beta-GlucogallinCAS# 13405-60-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

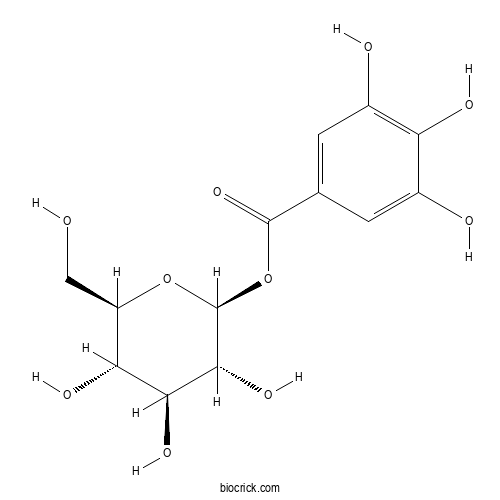
| Cas No. | 13405-60-2 | SDF | Download SDF |
| PubChem ID | 124021 | Appearance | Off-white powder |
| Formula | C13H16O10 | M.Wt | 332.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Gallotannin 1; 1-O-Galloyl β-D-glucose | ||
| Solubility | Soluble in DMSO and water | ||
| Chemical Name | [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] 3,4,5-trihydroxybenzoate | ||
| SMILES | C1=C(C=C(C(=C1O)O)O)C(=O)OC2C(C(C(C(O2)CO)O)O)O | ||
| Standard InChIKey | GDVRUDXLQBVIKP-HQHREHCSSA-N | ||
| Standard InChI | InChI=1S/C13H16O10/c14-3-7-9(18)10(19)11(20)13(22-7)23-12(21)4-1-5(15)8(17)6(16)2-4/h1-2,7,9-11,13-20H,3H2/t7-,9-,10+,11-,13+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Beta-glucogallin , a recently described Aldose reductase (AR) inhibitor, has antioxidant and anti-inflammatory activities. | |||||

beta-Glucogallin Dilution Calculator

beta-Glucogallin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0093 mL | 15.0466 mL | 30.0933 mL | 60.1866 mL | 75.2332 mL |
| 5 mM | 0.6019 mL | 3.0093 mL | 6.0187 mL | 12.0373 mL | 15.0466 mL |
| 10 mM | 0.3009 mL | 1.5047 mL | 3.0093 mL | 6.0187 mL | 7.5233 mL |
| 50 mM | 0.0602 mL | 0.3009 mL | 0.6019 mL | 1.2037 mL | 1.5047 mL |
| 100 mM | 0.0301 mL | 0.1505 mL | 0.3009 mL | 0.6019 mL | 0.7523 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,4,6-Trihydroxybenzaldehyde
Catalog No.:BCN9797
CAS No.:487-70-7
- (1R)-Chrysanthemolactone
Catalog No.:BCN9796
CAS No.:14087-70-8
- Methyl trans-cinnamate
Catalog No.:BCN9795
CAS No.:1754-62-7
- 2-Propylpyridine
Catalog No.:BCN9794
CAS No.:622-39-9
- 4',5,7-Trihydroxy 3,6,8-trimethoxyflavone
Catalog No.:BCN9793
CAS No.:57393-71-2
- Isoamyl butyrate
Catalog No.:BCN9792
CAS No.:106-27-4
- Ginkgotoxin hydrochloride
Catalog No.:BCN9791
CAS No.:3131-27-9
- Resokaempferol
Catalog No.:BCN9790
CAS No.:2034-65-3
- Kaempferol 3-robinoside 7-glucoside
Catalog No.:BCN9789
CAS No.:114924-89-9
- Ethyl phenylacetate
Catalog No.:BCN9788
CAS No.:101-97-3
- Quercetin 3-O-beta-D-glucosyl-(1->2)-rhamnoside
Catalog No.:BCN9787
CAS No.:143016-74-4
- 3-Methyl-1-butanol
Catalog No.:BCN9786
CAS No.:123-51-3
- 2-(2-Hydroxy-2-propyl)-5-methyl-5-vinyltetrahydrofuran
Catalog No.:BCN9799
CAS No.:60047-17-8
- 4-Hydroxy-6-methylcoumarin
Catalog No.:BCN9800
CAS No.:13252-83-0
- DL-2-Aminosuccinamic acid hydrate
Catalog No.:BCN9801
CAS No.:3130-87-8
- Quinidine sulfate
Catalog No.:BCN9802
CAS No.:50-54-4
- Stigmast-7-en-3-ol
Catalog No.:BCN9803
CAS No.:18525-35-4
- Gossypetin 3-methylether
Catalog No.:BCN9804
CAS No.:86749-51-1
- Sennoside A1
Catalog No.:BCN9805
CAS No.:66575-30-2
- Cimicifugic acid B
Catalog No.:BCN9806
CAS No.:205114-66-5
- Polygalacin D2
Catalog No.:BCN9807
CAS No.:66663-92-1
- DL-Phenylalanine
Catalog No.:BCN9808
CAS No.:150-30-1
- Isoedultin
Catalog No.:BCN9809
CAS No.:43043-08-9
- Quercetin 3-rutinoside 7-glucoside
Catalog No.:BCN9810
CAS No.:30311-61-6
Genome-Wide Analysis of Serine Carboxypeptidase-Like Acyltransferase Gene Family for Evolution and Characterization of Enzymes Involved in the Biosynthesis of Galloylated Catechins in the Tea Plant (Camellia sinensis).[Pubmed:32670320]
Front Plant Sci. 2020 Jun 25;11:848.
Tea (Camellia sinensis L.) leaves synthesize and concentrate a vast array of galloylated catechins (e.g., EGCG and ECG) and non-galloylated catechins (e.g., EGC, catechin, and epicatechin), together constituting 8%-24% of the dry leaf mass. Galloylated catechins account for a major portion of soluble catechins in tea leaves (up to 75%) and make a major contribution to the astringency and bitter taste of the green tea, and their pharmacological activity for human health. However, the catechin galloylation mechanism in tea plants is largely unknown at molecular levels. Previous studies indicated that glucosyltransferases and serine carboxypeptidase-like acyltransferases (SCPL) might be involved in the process. However, details about the roles of SCPLs in the biosynthesis of galloylated catechins remain to be elucidated. Here, we performed the genome-wide identification of SCPL genes in the tea plant genome. Several SCPLs were grouped into clade IA, which encompasses previously characterized SCPL-IA enzymes with an acylation function. Twenty-eight tea genes in this clade were differentially expressed in young leaves and vegetative buds. We characterized three SCPL-IA enzymes (CsSCPL11-IA, CsSCPL13-IA, CsSCPL14-IA) with galloylation activity toward epicatechins using recombinant enzymes. Not only the expression levels of these SCPLIA genes coincide with the accumulation of galloylated catechins in tea plants, but their recombinant enzymes also displayed beta-Glucogallin:catechin galloyl acyltransferase activity. These findings provide the first insights into the identities of genes encoding glucogallin:catechin galloyl acyltransferases with an active role in the biosynthesis of galloylated catechins in tea plants.
Protective Effect of beta-Glucogallin on Damaged Cataract Against Methylglyoxal Induced Oxidative Stress in Cultured Lens Epithelial Cells.[Pubmed:31811113]
Med Sci Monit. 2019 Dec 7;25:9310-9318.
BACKGROUND ss-glucogallin (GG) is one of the major plant polyphenolic antioxidants that have been associated with positive effects on human health and are crucial in the developing defense mechanism against the risk of diseases. However, reports on the protective mechanism of GG in lens epithelial cells are limited. MATERIAL AND METHODS ARPE-19 cells (a human retinal epithelial cell line) were exposed to methylglyoxal (MG) with or without GG to illuminate the protective role of GG in counteracting the cataract signaling. RESULTS Cells predisposed to MG demonstrated an increase in oxidative stress with augmented (P<0.01) inflammatory cytokines such as cyclooxygenase (COX)-2, chemokine receptor CXCR4, interleukin (IL)-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), and intercellular adhesion molecule 1 (ICAM-1) genes. In addition, the expression of aldose reductase (AR) was increased to 2-fold with accumulated sorbitol in MG exposed cells compared to control. On the other hand, cells exposed to MG evidenced a 3-fold increase in RAGE (receptor for advanced glycation end products) and a 2-fold increase in NF-kappaB (nuclear factor kappa-light-chain-enhancer of activated B cells) expression compared to control cells. Intriguingly, lens epithelial cells pre-treated with GG attenuated the reactive oxygen species levels with improved antioxidant enzymes. Simultaneously, the levels of AR and other inflammatory cytokines were observed in the levels closer to control cells in GG pre-treated cells. CONCLUSIONS Thus, the results of the present investigation show that GG may be a potential drug for the prevention of cataract development and progression.
Effective genome editing and identification of a regiospecific gallic acid 4-O-glycosyltransferase in pomegranate (Punica granatum L.).[Pubmed:31728198]
Hortic Res. 2019 Nov 8;6:123.
Pomegranate (Punica granatum L.) trees are woody perennials that bear colorful and nutritious fruits rich in phenolic metabolites, e.g., hydrolyzable tannins (HTs) and flavonoids. We here report genome editing and gene discovery in pomegranate hairy roots using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) (CRISPR/Cas9), coupled with transcriptome and biochemical analyses. Single guide RNAs (sgRNAs) were designed to target two UDP-dependent glycosyltransferases (UGTs), PgUGT84A23 and PgUGT84A24, which possess overlapping activities in beta-Glucogallin (a galloylglucose ester; biosynthetic precursor of HTs) biosynthesis. A unique accumulation of gallic acid 3-O- and 4-O-glucosides (galloylglucose ethers) was observed in the PgUGT84A23 and PgUGT84A24 dual CRISPR/Cas9-edited lines (i.e., ugt84a23 ugt84a24) but not the control (empty vector) or PgUGT84A23/PgUGT84A24 single edited lines (ugt84a23 or ugt84a24). Transcriptome and real-time qPCR analyses identified 11 UGTs with increased expression in the ugt84a23 ugt84a24 hairy roots compared to the controls. Of the 11 candidate UGTs, only PgUGT72BD1 used gallic acid as substrate and produced a regiospecific product gallic acid 4-O-glucoside. This work demonstrates that the CRISPR/Cas9 method can facilitate functional genomics studies in pomegranate and shows promise for capitalizing on the metabolic potential of pomegranate for germplasm improvement.
Standardized Emblica officinalis fruit extract inhibited the activities of alpha-amylase, alpha-glucosidase, and dipeptidyl peptidase-4 and displayed antioxidant potential.[Pubmed:31487036]
J Sci Food Agric. 2020 Jan 30;100(2):509-516.
BACKGROUND: Emblica officinalis, known as amla in Ayurveda, has been used as a folk medicine to treat numerous pathological conditions, including diabetes. However, the novel extract of E. officinalis fruit extract (amla fruit extract, AFE, Saberry(R)) containing 100 g kg(-1) beta-Glucogallin along with hydrolyzable tannins has not yet been extensively studied for its antidiabetic potential. OBJECTIVE: The aim of this study was to investigate the antidiabetic and antioxidant activities of AFE and its stability during gastric stress as well as its thermostability. METHODS: The effect of AFE on the inhibition of pancreatic alpha-amylase and salivary alpha-amylase enzymes was studied using starch and yeast alpha-glucosidase enzyme using 4-nitrophenyl alpha-d-glucopyranoside as substrate. Further, 2,2-diphenyl-1-picrylhydrazyl radical scavenging and reactive oxygen species inhibition assay was performed against AFE. RESULTS: AFE potently inhibited the activities of alpha-amylase and alpha-glucosidase in a concentration-dependent manner with half maximal inhibitory concentration (IC50 ) values of 135.70 mug mL(-1) and 106.70 mug mL(-1) respectively. Furthermore, it also showed inhibition of alpha-glucosidase (IC50 562.9 mug mL(-1) ) and dipeptidyl peptidase-4 (DPP-4; IC50 3770 mug mL(-1) ) enzyme activities. AFE is a potent antioxidant showing a free radical scavenging activity (IC50 2.37 mug mL(-1) ) and protecting against cellular reactive oxygen species (IC50 1.77 mug mL(-1) ), and the effects elicited could be attributed to its phytoconstituents. CONCLUSION: AFE showed significant gastric acid resistance and was also found to be thermostable against wet heat. Excellent alpha-amylase, alpha-glucosidase, and DPP-4 inhibitory activities of AFE, as well as antioxidant activities, strongly recommend its use for the management of type 2 diabetes mellitus. (c) 2019 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Identification of UDP glucosyltransferases from the aluminum-resistant tree Eucalyptus camaldulensis forming beta-glucogallin, the precursor of hydrolyzable tannins.[Pubmed:29775866]
Phytochemistry. 2018 Aug;152:154-161.
In the highly aluminum-resistant tree Eucalyptus camaldulensis, hydrolyzable tannins are proposed to play a role in internal detoxification of aluminum, which is a major factor inhibiting plant growth on acid soils. To understand and modulate the molecular mechanisms of aluminum detoxification by hydrolyzable tannins, the biosynthetic genes need to be identified. In this study, we identified and characterized genes encoding UDP-glucose:gallate glucosyltransferase, which catalyzes the formation of 1-O-galloyl-beta-d-glucose (beta-Glucogallin), the precursor of hydrolyzable tannins. By homology-based cloning, seven full-length candidate cDNAs were isolated from E. camaldulensis and expressed in Escherichia coli as recombinant N-terminal His-tagged proteins. Phylogenetic analysis classified four of these as UDP glycosyltransferase (UGT) 84A subfamily proteins (UGT84A25a, -b, UGT84A26a, -b) and the other three as UGT84J subfamily proteins (UGT84J3, -4, -5). In vitro enzyme assays showed that the UGT84A proteins catalyzed esterification of UDP-glucose and gallic acid to form 1-O-galloyl-beta-d-glucose, whereas the UGT84J proteins were inactive. Further analyses with UGT84A25a and -26a indicated that they also formed 1-O-glucose esters of other structurally related hydroxybenzoic and hydroxycinnamic acids with a preference for hydroxybenzoic acids. The UGT84A genes were expressed in leaves, stems, and roots of E. camaldulensis, regardless of aluminum stress. Taken together, our results suggest that the UGT84A subfamily enzymes of E. camaldulensis are responsible for constitutive production of 1-O-galloyl-beta-d-glucose, which is the first step of hydrolyzable tannin biosynthesis.


