3-Methyl-1-butanolCAS# 123-51-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123-51-3 | SDF | Download SDF |
| PubChem ID | 31260 | Appearance | Oil |
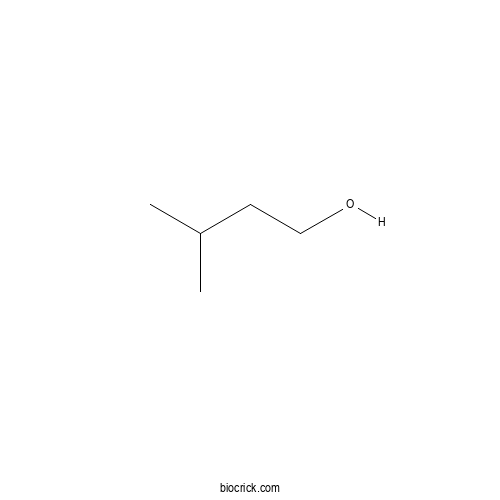
| Formula | C5H12O | M.Wt | 88.1 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-methylbutan-1-ol | ||
| SMILES | CC(C)CCO | ||
| Standard InChIKey | PHTQWCKDNZKARW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H12O/c1-5(2)3-4-6/h5-6H,3-4H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3‐Methyl‐1‐butanol is a volatile anti‐cyanobacterial and phytotoxic product of some Bacillus spp. | |||||

3-Methyl-1-butanol Dilution Calculator

3-Methyl-1-butanol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 11.3507 mL | 56.7537 mL | 113.5074 mL | 227.0148 mL | 283.7684 mL |
| 5 mM | 2.2701 mL | 11.3507 mL | 22.7015 mL | 45.403 mL | 56.7537 mL |
| 10 mM | 1.1351 mL | 5.6754 mL | 11.3507 mL | 22.7015 mL | 28.3768 mL |
| 50 mM | 0.227 mL | 1.1351 mL | 2.2701 mL | 4.5403 mL | 5.6754 mL |
| 100 mM | 0.1135 mL | 0.5675 mL | 1.1351 mL | 2.2701 mL | 2.8377 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,6,7-Trimethoxyflavone
Catalog No.:BCN9785
CAS No.:973-67-1
- Indole
Catalog No.:BCN9784
CAS No.:120-72-9
- Cinobufotenine
Catalog No.:BCN9783
CAS No.:60657-23-0
- Deacylgymnemic acid
Catalog No.:BCN9782
CAS No.:121686-42-8
- Cannabisin B
Catalog No.:BCN9781
CAS No.:144506-17-2
- (+/-)-Anabasine
Catalog No.:BCN9780
CAS No.:13078-04-1
- alpha-Hexylcinnamaldehyde
Catalog No.:BCN9779
CAS No.:101-86-0
- Calvatic acid
Catalog No.:BCN9778
CAS No.:54723-08-9
- 3,4-Dimethoxyacetophenone
Catalog No.:BCN9777
CAS No.:1131-62-0
- 3,5-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN9776
CAS No.:4319-02-2
- Nortrachelogenin-5'-C-beta-glucoside
Catalog No.:BCN9775
CAS No.:858127-39-6
- Glucofrangulin A
Catalog No.:BCN9774
CAS No.:21133-53-9
- Quercetin 3-O-beta-D-glucosyl-(1->2)-rhamnoside
Catalog No.:BCN9787
CAS No.:143016-74-4
- Ethyl phenylacetate
Catalog No.:BCN9788
CAS No.:101-97-3
- Kaempferol 3-robinoside 7-glucoside
Catalog No.:BCN9789
CAS No.:114924-89-9
- Resokaempferol
Catalog No.:BCN9790
CAS No.:2034-65-3
- Ginkgotoxin hydrochloride
Catalog No.:BCN9791
CAS No.:3131-27-9
- Isoamyl butyrate
Catalog No.:BCN9792
CAS No.:106-27-4
- 4',5,7-Trihydroxy 3,6,8-trimethoxyflavone
Catalog No.:BCN9793
CAS No.:57393-71-2
- 2-Propylpyridine
Catalog No.:BCN9794
CAS No.:622-39-9
- Methyl trans-cinnamate
Catalog No.:BCN9795
CAS No.:1754-62-7
- (1R)-Chrysanthemolactone
Catalog No.:BCN9796
CAS No.:14087-70-8
- 2,4,6-Trihydroxybenzaldehyde
Catalog No.:BCN9797
CAS No.:487-70-7
- beta-Glucogallin
Catalog No.:BCN9798
CAS No.:13405-60-2
Determination of volatile compounds during deterioration of African opaque beer using a stir bar sorptive extraction technique and gas chromatography-high resolution mass spectrometry.[Pubmed:33294852]
Curr Res Food Sci. 2020 Nov 11;3:256-267.
Opaque beer traditional to African communities undergoes quick deterioration and is consumed within 7 days of its production. The current study has utilized a stir bar sorptive extraction technique followed by GC-HRT determination to trace variations of 84 volatile compounds in four opaque beers commonly brewed in South Africa over the 7-day shelf life period. The major fruity esters were observed to increase up to Day 4 and eventually decreasing until Day 7 where their levels were finally lower than Day 1. Aldehydes reduced drastically and were less than 50% on Day 2 and becoming almost undetectable at Day 7. The common beer alcohols (phenylethyl alcohol and 3-Methyl-1-butanol) decreased during beer shelf life while phenolics with undesirable medicinal tastes (creosol and p-cresol) increased up to 24-fold by Day 7. This study might open future research perspectives around opaque beer traditional to African rural communities.
Key aroma compounds of Chinese dry-cured Spanish mackerel (Scomberomorus niphonius) and their potential metabolic mechanisms.[Pubmed:33097327]
Food Chem. 2020 Oct 16:128381.
The key aroma compounds of six commercially available dry-cured Spanish mackerel (Scomberomorus niphonius, DCSM) were identified using electronic nose (E-nose), gas chromatography-olfactometry (GC-O), and two-dimensional gas chromatography-time-of-flight mass spectrometry (GC x GC-TOFMS). A total of 38-55 aroma compounds were identified, and 21-26 of them, which presented high flavor dilution factors based on aroma extract dilution analysis, were quantified. Lastly, 9-14 key aroma compounds with high odor-active value, including 3-methyl-1-butanal, octanal, 1-octen-3-ol, nonanal, cis-4-decenal, ethyl caproate, (E)-2-octenal, (Z)-2-nonenal decanal, 3-Methyl-1-butanol, 1-heptanol, 3-octanone, 2-octanol, and 6-methyl-5-hepten-2-one, were identified as the key aroma contributors in DCSM. Results also indicated that a longer dry-curing time would promote the generation of aroma compounds. The metabolism analysis implied that the auto-oxidation/oxidation of unsaturated fatty acids, such as oleic and linoleic acid, and the enzymatic degradation of l-leucine might be potential metabolic mechanisms.
Improved flavor profiles of red pitaya (Hylocereus lemairei) wine by controlling the inoculations of Saccharomyces bayanus and Metschnikowia agaves and the fermentation temperature.[Pubmed:33087960]
J Food Sci Technol. 2020 Dec;57(12):4469-4480.
The effects of the inoculation method of Saccharomyces bayanus BV818 and non-Saccharomyces yeast Metschnikowia agaves P3-3 and the fermentation temperature on the volatile profiles of red pitaya wine were investigated in the present study. Although the growth of P3-3 was inhibited by BV818 in the mixed inoculations, simultaneous and sequential inoculations promoted the production of seven volatiles, including higher alcohols (propan-1-ol, 3-Methyl-1-butanol and phenethyl alcohol), esters (ethyl decanoate and diethyl succinate), acid (2-ethylhexanoic acid), and ketone (acetoin). Sequential inoculation produced the largest total content of volatile compounds and exhibited the best in the global aroma. The red pitaya wine produced in different inoculations can be separated by its main volatile components. Furthermore, the highest total content was yielded at 25 degrees C for alcohols and at 21 degrees C for esters and acids. Within an experimental range of 17 degrees C to 29 degrees C, the contents of benzaldehyde and acetoin decreased with the increase in temperature, whereas the change in 4-ethyl-2-methoxyphenol content was the opposite. The similarly high total contents of volatiles and global aroma score were yielded via sequential inoculation at 21 degrees C and 25 degrees C. Therefore, the desired red pitaya wine can be effectively produced by modulating the inoculation method and fermentation temperature.
Bioactivity of volatile organic compounds by Aureobasidium species against gray mold of tomato and table grape.[Pubmed:33067644]
World J Microbiol Biotechnol. 2020 Oct 17;36(11):171.
Aureobasidium strains isolated from diverse unconventional environments belonging to the species A. pullulans, A. melanogenum, and A. subglaciale were evaluated for Volatile Organic Compounds (VOCs) production as a part of their modes of action against Botrytis cinerea of tomato and table grape. By in vitro assay, VOCs generated by the antagonists belonging to the species A. subglaciale showed the highest inhibition percentage of the pathogen mycelial growth (65.4%). In vivo tests were conducted with tomatoes and grapes artificially inoculated with B. cinerea conidial suspension, and exposed to VOCs emitted by the most efficient antagonists of each species (AP1, AM10, AS14) showing that VOCs of AP1 (A. pullulans) reduced the incidence by 67%, partially confirmed by the in vitro results. Conversely, on table grape, VOCs produced by all the strains did not control the fungal incidence but were only reducing the infection severity (< 44.4% by A. pullulans; < 30.5% by A. melanogenum, and A. subglaciale). Solid-phase microextraction (SPME) and subsequent gas chromatography coupled to mass spectrometry identified ethanol, 3-Methyl-1-butanol, 2-methyl-1-propanol as the most produced VOCs. However, there were differences in the amounts of produced VOCs as well as in their repertoire. The EC50 values of VOCs for reduction of mycelial growth of B. cinerea uncovered 3-Methyl-1-butanol as the most effective compound. The study demonstrated that the production and the efficacy of VOCs by Aureobasidium could be directly related to the specific species and pathosystem and uncovers new possibilities for searching more efficient VOCs producing strains in unconventional habitats other than plants.
Characterization of the key aroma compounds in Yunnan goat milk cake using a sensory-directed flavor analysis.[Pubmed:33063315]
J Food Sci. 2020 Nov;85(11):3981-3997.
To identify the key aroma compounds in Yunnan goat milk cake, seven varieties of milk cake samples were subjected to sensory analysis and gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), aroma recombination, omission, and addition tests. The GC-MS results revealed 53 compounds with aroma characteristics in all the samples. A further comparison of odor activity values and aroma intensities (AI) revealed 25 of these compounds as the initial key aroma compounds. The contributions of these key aroma compounds to the sensory attributes were determined using a partial least squares regression. Of these compounds, 2-heptanone and 2-nonanone were closely related to the "milky" and "cheesy" attributes and were highly abundant in the samples from Kunming. Fatty acids, including butanoic acid, hexanoic acid, octanoic acid, and decanoic acid, were the most abundant compounds detected in the milk cakes. These fatty acids were closely related to the "rancid" and "animalic (goat)" attributes and were largely detected in the samples from Dali Dengchuan and Dali Xiaguan. Sensory-directed aroma recombination, omission, and addition tests further validated the important contributions of ethyl butyrate, benzaldehyde, 3-Methyl-1-butanol, 2-heptanone, hexanoic acid, and octanoic acid to the overall sensory properties. Moreover, ethyl butyrate, benzaldehyde, and 2-heptanone, when added, had evident inhibitory or masking effects on the AI of "sour," "rancid," and "animalic (goat)" attributes. PRACTICAL APPLICATION: Goat milk cake is a popular acid-curd cheese in Yunnan, China, however, our limited knowledge to its key aroma compounds restricts its development and industrial production. In this study, a sensory-directed flavor analysis was used to characterized the key aroma compounds of Yunnan goat milk cake, which will help to enhance our understanding on the flavor profile of Yunnan goat milk cake and provide a reference for optimizing the flavor feature and organoleptic quality of this fresh goat cheese.
Volatile organic compounds produced by Aureobasidium pullulans induce electrolyte loss and oxidative stress in Botrytis cinerea and Alternaria alternata.[Pubmed:33049328]
Res Microbiol. 2020 Oct 10. pii: S0923-2508(20)30091-7.
Aureobasidium pullulans is a yeast-like fungus that produces volatile organic compounds (VOCs) with antifungal properties. VOCs have the potential to trigger the production of intracellular reactive oxygen species (ROS), lipid peroxidation and electrolyte loss in microorganisms. The relationship among A. pullulans VOCs, induced ROS accumulation and electrolyte leakage was investigated in Botrytis cinerea and Alternaria alternata in vitro. Exposure to a mixture of A. pullulans VOCs: ethanol, 2-methyl-1-propanol, 3-Methyl-1-butanol and 2-phenylethanol, resulted in electrolyte leakage in both B. cinerea and A. alternata. Fluorescence microscopy using 2',7'-dichlorofluorescein diacetate indicated triggered ROS accumulation in exposed fungal mycelia and the presence of the superoxide radical was evident by intense red fluorescence with dihydroethidium. Partial inhibition of enzymes of the mitochondrial respiratory chain complex I of B. cinerea and A. alternata by pre-treatment with rotenone reduced ROS accumulation in hypha exposed to A. pullulans VOCs and reversed the VOCs inhibition of fungal growth. Scanning electron micrographs revealed that B. cinerea and A. alternata hypha exposed to A. pullulans VOCs had altered cell wall structures. Our findings give insights into the potential mechanisms involved in the antifungal properties of A. pullulans in the suppression of B. cinerea and A. alternata growth in vitro.
Development and Validation of an Analytical Method for Volatiles with Endogenous Production in Putrefaction and Submersion Situations.[Pubmed:33031530]
J Anal Toxicol. 2020 Oct 8. pii: 5919708.
A group of 16 volatile substances (ethyl acetate, 2-propanol, 1-propanol, methanol, acetone, ethanol, acetaldehyde, diethyl ether, methyl ethyl ketone, 1-butanol, 2-butanol, t-butanol, isobutanol, 2-methyl-1-butanol, 3-Methyl-1-butanol, 1-pentanol) were qualitatively and quantitatively analyzed through a method developed for volatiles with endogenous production in putrefaction and submersion situations. The method was validated for blood, urine and vitreous humor, using a Varian 450-GC gas chromatograph with a flame ionization detector coupled to a headspace injector (HS-GC-FID). The vials were prepared by diluting 100 microL of the sample of interest in 1 mL internal standard (acetonitrile 100 mg/L), using two capillary columns (VF-624ms and VF-5ms) with different polarities to ensure that all test compounds would be properly identified and undoubtedly distinguished from the rest. All volatiles were studied in a range of 50-2000 mg/L in terms of selectivity/specificity, detection and quantification limits, linearity and calibration model, precision, accuracy, bias, robustness and stability according to the Scientific Working Group for Forensic Toxicology. Detection and quantification limits were between 1-8 mg/L and 4-24 mg/L, respectively, with coefficient of variation values under 10% in bias studies and in intermediate precision studies for most substances. The developed method was applied to real cases to test the method.
Monitoring of infection volatile markers using CMOS-based luminescent bioreporters.[Pubmed:32887066]
Talanta. 2020 Nov 1;219:121333.
Over the past two decades, whole-cell biosensors (WCBs) have been widely used in the environmental field, with only few applications proposed for use in agricultural. This study describes the development and optimization of a WCB for the detection of volatile organic compounds (VOCs) that is produced specifically by infected potato tubers. First, the effect of calcium-alginate matrix formation (beads vs. tablets) on the membrane uniformity and sensing efficiency was evaluated. Then, important parameters in the immobilization process were examined for their effect on the sensitivity to the presence of VOCs. The highest sensitivity to the target VOC was obtained by 20 min polymerization of bacterial suspension with optical density of 0.2 at 600 nm, dissolved in low-viscosity sodium alginate (1.5% w/v) and exposure to VOC at 4 degrees C. After optimization, the lowest limit of detection for three infection-sourced VOCs (nonanal, 3-Methyl-1-butanol, and 1-octen-3-ol) was 0.17-, 2.03-, and 2.09-mg/L, respectively, and the sensor sensitivity was improved by 8.9-, 3.1- and 2-fold, respectively. Then, the new optimized immobilization protocol was implemented for the CMOS-based application, which increased the sensor sensitivity to VOC by 3-fold during real-time measurement. This is the first step in creating a sensor for real-time monitoring of crop quality by identifying changes in VOC patterns.
The Use of Sour and Sweet Whey in Producing Compositions with Pleasant Aromas Using the Mold Galactomyces geotrichum: Identification of Key Odorants.[Pubmed:32865406]
J Agric Food Chem. 2020 Sep 30;68(39):10799-10807.
Fermented products with a pleasant aroma and with strong honey, rose, and fruit odor notes were developed through the biotransformation of a medium containing sour or sweet whey with the addition of l-phenylalanine by the Galactomyces geotrichum mold. In order to obtain the strong honey-rose aroma, G. geotrichum strains were screened and fermentation conditions were optimized to achieve a preferable ratio (>1) of phenylacetaldehyde to 2-phenylethanol by the Ehrlich pathway. This allowed post-fermentation products with the ratio of concentrations of phenylacetaldehyde to 2-phenylethanol being 1.7:1. Additionally, the use of gas chromatography-olfactometry (GC-O) analysis and the calculation of odor activity values (OAVs) allowed 10 key odorants to be identified in post-fermentation products. The highest OAVs were found for phenylacetaldehyde with a honey odor in both sour and sweet whey cultures (3010 and 1776, respectively). In the variant with sour whey, the following compounds with the highest OAVs were 3-Methyl-1-butanol (131), 3-(methylthio)-propanal (119), 3-methylbutanal (90), dimethyl trisulfide (71), 2,3-butanedione (37), and 2-phenylethanol (29). In the post-fermentation product with sweet whey, the following compounds with the highest OAVs were 3-(methylthio)-propanal (112), dimethyl trisulfide (69), and 2,3-butanedione (41).
Trichoderma Volatile Organic Compounds as a Biofumigation Tool against Late Blight Pathogen Phytophthora infestans in Postharvest Potato Tubers.[Pubmed:32790355]
J Agric Food Chem. 2020 Aug 5;68(31):8163-8171.
We tested the ability of 14 strains of Trichoderma to emit volatile compounds that decreased or stopped the growth of Phytophthora infestans. Volatile organic compounds (VOCs) emitted from Trichoderma strains designated T41 and T45 inhibited the mycelial growth of P. infestans grown on a laboratory medium by 80 and 81.4%, respectively, and on potato tubers by 93.1 and 94.1%, respectively. Using the DNA sequence analysis of the translation elongation factor region, both Trichoderma strains were identified as Trichoderma atroviride. VOCs emitted by the strains were analyzed, and 39 compounds were identified. The most abundant compounds were 3-Methyl-1-butanol, 6-pentyl-2-pyrone, 2-methyl-1-propanol, and acetoin. Electron microscopy of the hyphae treated with T. atroviride VOCs revealed serious morphological and ultrastructural damages, including cell deformation, collapse, and degradation of cytoplasmic organelles. To our knowledge, this is the first report describing the ability of Trichoderma VOCs to suppress the growth of the late blight potato pathogen.
Dry-Hopping to Modify the Aroma of Alcohol-Free Beer on a Molecular Level-Loss and Transfer of Odor-Active Compounds.[Pubmed:32657584]
J Agric Food Chem. 2020 Aug 12;68(32):8602-8612.
There are mainly two options for the dealcoholization of beer: evaporation of ethanol by heat treatment, whereby desired aroma-active compounds are also removed, and stopped fermentation that leads to beers still containing high amounts of unfermented sugar in parallel with lower amounts of aroma-active fermentation products. Thus, dry-hopping could be an opportunity to compensate for these aroma deficiencies. Therefore, following the sensomics approach, odorants were characterized in dry-hopped (Hallertauer Mandarina Bavaria, Hallertauer Cascade, or Hallertauer Mittelfruh) top- and bottom-fermented alcohol-free beers either after thermal dealcoholization or stopped fermentation. Twenty-three odorants were quantitated via stable isotope dilution analysis, and odor activity values (OAVs; ratio of concentration to odor threshold) were calculated. Thermally dealcoholized samples showed high losses (up to 100%) of key odorants like 3-Methyl-1-butanol or 3-methylbutyl acetate. During stopped fermentation, aroma compounds like ethyl butanoate or 2-phenylethanol were formed in relevant concentrations, leading to OAVs >/= 1, but the amounts were significantly lower compared to beers with normal alcohol contents. For hop-derived odorants (linalool, geraniol, myrcene, and esters), transfer rates between 20 and 90% were found, leading to OAVs >/= 1 in beer. Furthermore, hop addition apparently induced the formation of ethyl esters of hop-derived monocarboxylic acids.
Contribution of the retronasal odor of soy sauce to salt reduction.[Pubmed:32654181]
J Food Sci. 2020 Aug;85(8):2523-2529.
The characteristic odor of soy sauce has been reported to enhance saltiness. However, soy sauce is used not only as a sauce that is added directly to food, but also as a seasoning. In addition, some of the aromatic compounds that contribute to the soy sauce odor change during cooking or heating. In the present study, the effects of the retronasal odor of uncooked and cooked soy sauce on the enhancement of saltiness and palatability of a low-salt solution were sensory evaluated. A probit analysis indicated that the saltiness-enhancing effect of the odor of 15% uncooked soy sauce was lost by heating. The odors of soy sauce boiled for 10 min (cooked SS) and the residue of soy sauce heated at 200 degrees C for 1 min improved the palatability of the low-salt solution. Gas chromatography (GC) analyses, namely, GC-olfactometry and GC-mass spectrometry, showed that one active candidate aromatic component of soy sauce contributing to saltiness enhancement was 3-Methyl-1-butanol (3-Me-BuOH). The saltiness-enhancing effects of cooked SS could be restored by adding 3-Me-BuOH, as assessed by the sensory evaluation. These data demonstrated that 3-Me-BuOH contributes to saltiness enhancement. PRACTICAL APPLICATION: We found that the odor of cooked soy sauce could improve the palatability of low-salt food. Although the saltiness-enhancing effect provided by the odor of uncooked soy sauce was lost, the saltiness-enhancing effect of the odor of cooked soy sauce can be partially restored by adding 3-methyl butanol. Thus, not only the odor of unheated soy sauce but also the odor of heated soy sauce following addition of 3-methyl butanol may be useful for developing palatable salt-reduced food.


