5,6,7-TrimethoxyflavoneCAS# 973-67-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 973-67-1 | SDF | Download SDF |
| PubChem ID | 442583 | Appearance | Powder |
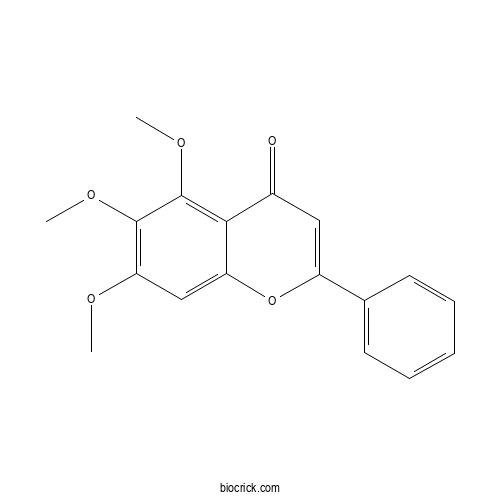
| Formula | C18H16O5 | M.Wt | 312.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5,6,7-trimethoxy-2-phenylchromen-4-one | ||
| SMILES | COC1=C(C(=C2C(=C1)OC(=CC2=O)C3=CC=CC=C3)OC)OC | ||
| Standard InChIKey | HJNJAUYFFFOFBW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O5/c1-20-15-10-14-16(18(22-3)17(15)21-2)12(19)9-13(23-14)11-7-5-4-6-8-11/h4-10H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 2. | |||||

5,6,7-Trimethoxyflavone Dilution Calculator

5,6,7-Trimethoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.202 mL | 16.0102 mL | 32.0205 mL | 64.041 mL | 80.0512 mL |
| 5 mM | 0.6404 mL | 3.202 mL | 6.4041 mL | 12.8082 mL | 16.0102 mL |
| 10 mM | 0.3202 mL | 1.601 mL | 3.202 mL | 6.4041 mL | 8.0051 mL |
| 50 mM | 0.064 mL | 0.3202 mL | 0.6404 mL | 1.2808 mL | 1.601 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3202 mL | 0.6404 mL | 0.8005 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Indole
Catalog No.:BCN9784
CAS No.:120-72-9
- Cinobufotenine
Catalog No.:BCN9783
CAS No.:60657-23-0
- Deacylgymnemic acid
Catalog No.:BCN9782
CAS No.:121686-42-8
- Cannabisin B
Catalog No.:BCN9781
CAS No.:144506-17-2
- (+/-)-Anabasine
Catalog No.:BCN9780
CAS No.:13078-04-1
- alpha-Hexylcinnamaldehyde
Catalog No.:BCN9779
CAS No.:101-86-0
- Calvatic acid
Catalog No.:BCN9778
CAS No.:54723-08-9
- 3,4-Dimethoxyacetophenone
Catalog No.:BCN9777
CAS No.:1131-62-0
- 3,5-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN9776
CAS No.:4319-02-2
- Nortrachelogenin-5'-C-beta-glucoside
Catalog No.:BCN9775
CAS No.:858127-39-6
- Glucofrangulin A
Catalog No.:BCN9774
CAS No.:21133-53-9
- DL-Tyrosine
Catalog No.:BCN9773
CAS No.:556-03-6
- 3-Methyl-1-butanol
Catalog No.:BCN9786
CAS No.:123-51-3
- Quercetin 3-O-beta-D-glucosyl-(1->2)-rhamnoside
Catalog No.:BCN9787
CAS No.:143016-74-4
- Ethyl phenylacetate
Catalog No.:BCN9788
CAS No.:101-97-3
- Kaempferol 3-robinoside 7-glucoside
Catalog No.:BCN9789
CAS No.:114924-89-9
- Resokaempferol
Catalog No.:BCN9790
CAS No.:2034-65-3
- Ginkgotoxin hydrochloride
Catalog No.:BCN9791
CAS No.:3131-27-9
- Isoamyl butyrate
Catalog No.:BCN9792
CAS No.:106-27-4
- 4',5,7-Trihydroxy 3,6,8-trimethoxyflavone
Catalog No.:BCN9793
CAS No.:57393-71-2
- 2-Propylpyridine
Catalog No.:BCN9794
CAS No.:622-39-9
- Methyl trans-cinnamate
Catalog No.:BCN9795
CAS No.:1754-62-7
- (1R)-Chrysanthemolactone
Catalog No.:BCN9796
CAS No.:14087-70-8
- 2,4,6-Trihydroxybenzaldehyde
Catalog No.:BCN9797
CAS No.:487-70-7
Repurposing mosloflavone/5,6,7-trimethoxyflavone-resveratrol hybrids: Discovery of novel p38-alpha MAPK inhibitors as potent interceptors of macrophage-dependent production of proinflammatory mediators.[Pubmed:31310917]
Eur J Med Chem. 2019 Oct 15;180:253-267.
Herein, we address repurposing hybrids of mosloflavone or 5,6,7-Trimethoxyflavone with amide analogs of resveratrol from anticancer leads to novel potent anti-inflammatory chemical entities. To unveil the potent anti-inflammatory molecules, biological evaluations were initiated in LPS-induced RAW 264.7 macrophages at 1muM concentration. Promising compounds were further evaluated at various concentrations. Multiple proinflammatory mediators were assessed including NO, PGE2, IL-6, TNF-alpha and IL-1beta. Compound 5z inhibited the induced production of NO, PGE2, IL-6, TNF-alpha and IL-1beta at the low 1muM concentration by 44.76, 35.71, 53.48, 29.39 and 41.02%, respectively. Compound 5z elicited IC50 values as low as 2.11 and 0.98muM against NO and PGE2 production respectively. Compounds 5q and 5g showed potent submicromolar IC50 values of 0.31 and 0.59muM respectively against PGE2 production. Reverse docking of compound 5z suggested p38-alpha MAPK, which is a key signaling molecule within the pathways controlling the transcription of proinflammatory mediators, as the molecular target. Biochemical testing confirmed these compounds as p38-alpha MAPK inhibitors explaining its potent inhibition of proinflammatory mediators' production. Collectively, the results presented 5z as a promising compound for further development of anti-inflammatory agents for treatment of macrophages-and/or immune mediated inflammatory diseases.
Natural products hybrids: 3,5,4'-Trimethoxystilbene-5,6,7-trimethoxyflavone chimeric analogs as potential cytotoxic agents against diverse human cancer cells.[Pubmed:30396104]
Eur J Med Chem. 2019 Jan 1;161:559-580.
Cancer still represents a major global health problem. All currently available anticancer agents have disadvantages like resistance or side effects. Therefore, introduction of novel anticancer agents is needed. Intrigued by the high success rate for natural products-based drug discovery, we designed and synthesized antiproliferative chemical entities as hybrids of two natural products; 3,5,4'-trimethoxystilbene and 5,6,7-Trimethoxyflavone. To probe the spectrum of the synthesized compounds, in vitro evaluation was conducted against nine panels representing major cancer diseases. The results revealed the hybrid analogs 4f, 4h, 4k and 4q as promising broad-spectrum anticancer lead compounds eliciting high growth inhibition of several cell lines representing multiple cancers diseases. Evaluation of the promising lead compounds against normal human cell lines suggested a selective cytotoxic effect on cancer cells. Mechanistic investigation of the cytotoxic activity of compound 4f in human cervical cancer HeLa cells showed that it triggers cell death through induction of apoptosis. As a whole, this study presents the natural products hybrid analogs 4f, 4h, 4k and 4q as potential lead compounds for further development of novel anticancer therapeutics.
Synthesis of novel flavone derivatives possessing substituted benzamides and their biological evaluation against human cancer cells.[Pubmed:27503682]
Bioorg Med Chem Lett. 2016 Sep 1;26(17):4170-3.
Baicalein is a well-known flavone derivative that possesses diverse biological properties, such as anticancer, antioxidant and anti-inflammatory activities. Numerous baicalein derivatives, including 5,6,7-Trimethoxyflavone, have been synthesized with the aim of enhancing its inherent biological activities. In the present work, new flavones, possessing an N-aroylamine-substituent on the B-ring, were synthesized to improve the cytotoxicity of baicalein and 5,6,7-Trimethoxyflavone against human cancer cell lines. The majority of the flavones synthesized exhibited greater cytotoxicity than baicalein and 5,6,7-Trimethoxyflavone against HepG2 and MCF-7 cells. Among them, compounds 5n, possessing a 3-methoxybenzoylamino group, exhibited great cytotoxic effects on HepG2 (GI50=7.06muM) and MCF-7 (GI50=7.67muM) cells. In contrast, N-aroylamine-substituted 5-hydroxy-6,7-dimethoxyflavone derivatives showed greater cytotoxicity against MCF-7 than HepG2 cells, indicating that the replacement of a 5-methoxy group on the A-ring with a 5-hydroxy group has a marked influence on the cytotoxicity profile.
Simultaneous Quantification of Five Bioactive Flavonoids in High Altitude Plant Actinocarya tibetica by LC-ESI-MS/MS.[Pubmed:26268971]
J AOAC Int. 2015 Jul-Aug;98(4):907-12.
An LC/MS method has been developed for the simultaneous quantification of five flavonoids, i.e., mosloflavone, negletein, gardenin B, 5-methoxy-6,7-methylenedioxyflavone, and 5,6,7-Trimethoxyflavone, in different ultrasound assisted solvent extracts of Actinocarya tibetica. The chromatographic separation was achieved on a Chromolith Speed ROD RP-18e column with gradient elution using methanol and 0.1% formic acid in water. The calibration curves of all five analytes showed good linearity (R2>0.991). Accuracy and precision were within the required limits. The developed method could serve as an effective method for QC of A. tibetica. The investigated compounds were determined simultaneously for the first time in A. tibetica or any other plant.
Design, synthesis and evaluation of novel 5,6,7-trimethoxyflavone-6-chlorotacrine hybrids as potential multifunctional agents for the treatment of Alzheimer's disease.[Pubmed:25724825]
Bioorg Med Chem Lett. 2015 Apr 1;25(7):1541-5.
A series of 5,6,7-Trimethoxyflavone-6-chlorotacrine hybrids were designed, synthesized and evaluated as multifunctional agents for the treatment of Alzheimer's disease (AD). The results showed that the target compounds exhibited good acetylcholinesterase (AChE) inhibitory potencies, high selectivity toward AChE over butyrylcholinesterase (BuChE), potential antioxidant activities and significant inhibitory potencies of self-induced beta-amyloid peptide (Abeta) aggregation. In particular, compound 14c had the strongest AChE inhibitory activity with IC50 value of 12.8 nM, potent inhibition of self-induced Abeta1-42 aggregation with inhibition ratio of 33.8% at 25 muM. Moreover, compound 14c acted as an antioxidant, as well as a neuroprotectant. Furthermore, 14c could cross the blood-brain barrier (BBB) in vitro. The results showed that compound 14c might be a potential multifunctional candidate for the treatment of AD.
One new flavonoid from Oroxylum indicum.[Pubmed:25675364]
Nat Prod Res. 2015;29(19):1828-32.
One new flavonoid, 5,6,7-Trimethoxyflavone-8-O-beta-D-glucopyranoside (1), along with six known compounds 2-7, was isolated from Oroxylum indicum. Their structures were determined on the basis of spectral data. The antibacterial activities of compounds 1-4 were studied. Compounds 1 and 3 showed medium antibacterial activity against Staphylococcus aureus with MIC/MBC at 32-128 mug/ml.
5,6,7-trimethoxyflavone suppresses pro-inflammatory mediators in lipopolysaccharide-induced RAW 264.7 macrophages and protects mice from lethal endotoxin shock.[Pubmed:24161485]
Food Chem Toxicol. 2013 Dec;62:847-55.
5,6,7-Trimethoxyflavone (TMF), methylations of the hydroxyl groups of oroxylin A or baicalein, was found to significantly inhibit the productions of nitric oxide (NO) and prostaglandin E2 (PGE2) in lipopolysaccharide (LPS)-treated RAW 264.7 macrophages. However, no report has been issued on the anti-inflammatory potential of TMF and the underlying molecular mechanism. In the present study, we investigated the anti-inflammatory effects of TMF in LPS-induced RAW 264.7 macrophages and LPS-induced septic shock in mice. TMF dose-dependently inhibits iNOS and COX-2 at the protein, mRNA, and promoter binding levels and that these inhibitions cause attendant decreases in the productions of NO and PGE2. TMF inhibits the productions and mRNA expressions of tumor necrosis factor-alpha (TNF-alpha), interleukin (IL)-1beta, and IL-6 induced by LPS. Furthermore, TMF suppress the transcriptional activity of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1), and nuclear translocations of NF-kappaB, AP-1, and signal transducer and activator of transcription 1/3 (STAT1/3). Pretreatment with TMF increase the survival rate of mice with LPS-induced endotoxemia and reduced the serum levels of cytokines. Taken together, these findings suggest that TMF down-regulates the expressions of the pro-inflammatory iNOS, COX-2, TNF-alpha, IL-1beta, and IL-6 genes in macrophages by interfering with the activation of NF-kappaB, AP-1, and STAT1/3.
4'-bromo-5,6,7-trimethoxyflavone represses lipopolysaccharide-induced iNOS and COX-2 expressions by suppressing the NF-kappaB signaling pathway in RAW 264.7 macrophages.[Pubmed:22101131]
Bioorg Med Chem Lett. 2012 Jan 1;22(1):700-5.
The regulations of the NO and PGE(2) productions are research topics of interest in the field of anti-inflammatory drug development. In the present study, 5,6,7-trimethoxy- and 5,6,7-trihydroxyflavones 3a-3g were synthesized from cinnamic acid derivatives. In particular, 4'-bromo-5,6,7-Trimethoxyflavone (3b) most potently inhibited the productions of NO and PGE(2) in LPS-treated RAW 264.7 cells (IC(50)=14.22 +/- 1.25 and 10.98 +/- 6.25 muM, respectively), and these inhibitory effects were more potent than those of oroxylin A or baicalein. Consistent with these findings, 3b concentration-dependently reduced the LPS-induced expressions of iNOS and COX-2 at the protein and mRNA levels. In addition, the release of TNF-alpha, IL-6, and IL-1beta and the mRNA expressions of these cytokines were reduced by 3b in a concentration-dependent manner. Furthermore, 3b attenuated the LPS-induced transcriptional activities of NF-kappaB and this was accompanied by parallel reductions in the degradation and phosphorylation of IkappaB-alpha, and consequently by a decrease in the nuclear translocation of the p65 subunit of NF-kappaB. Taken together, these results suggest that suppressions of the expressions of iNOS, COX-2, TNF-alpha, IL-6, and IL-1beta via NF-kappaB inactivation are responsible for the anti-inflammatory effects of 3b.
Studies on the antimicrobial activity and brine shrimp toxicity of Zeyheria tuberculosa (Vell.) Bur. (Bignoniaceae) extracts and their main constituents.[Pubmed:19450272]
Ann Clin Microbiol Antimicrob. 2009 May 18;8:16.
BACKGROUND: Due to the indiscriminate use of antimicrobial drugs, the emergence of human pathogenic microorganisms resistant to major classes of antibiotics has been increased and has caused many clinical problems in the treatment of infectious diseases. Thus, the aim of this study was to evaluate for the first time the in vitro antimicrobial activity and brine shrimp lethality of extracts and isolated compounds from Zeyheria tuberculosa (Vell.) Bur., a species used in Brazilian folk medicine for treatment of cancer and skin diseases. METHODS: Using the disc diffusion method, bioautography assay and brine shrimp toxicity test (Artemia salina Leach), we studied the antimicrobial activity and lethality of extracts and isolated compounds against three microorganisms strains, including Gram-positive (Staphylococcus aureus) and Gram-negative (Pseudomonas aeruginosa) bacteria and yeasts (Candida albicans). RESULTS: In this study, the extracts inhibited S. aureus (8.0 +/- 0.0 to 14.0 +/- 0.0 mm) and C. albicans (15.3 +/- 0.68 to 25.6 +/- 0.4 mm) growth. In the brine shrimp test, only two of them showed toxic effects (LC50 29.55 to 398.05 microg/mL) and some extracts were non-toxic or showed weak lethality (LC50 705.02 to > 1000 microg/mL). From these extracts, four flavones [5,6,7,8-tetramethoxyflavone (1), 5,6,7-Trimethoxyflavone (2), 4'-hydroxy-5,6,7,8-tetramethoxyflavone (3), and 4'-hydroxy-5,6,7-Trimethoxyflavone (4)] were isolated through bioassay-guided fractionation and identified based on the 1D and 2D NMR spectral data. By bioautography assays, compounds 1 [S. aureus (16.0 +/- 0.0 mm) and C. albicans (20.0 +/- 0.0 mm)] and 3 [S. aureus (10.3 +/- 0.6 mm) and C. albicans (19.7 +/- 0.6 mm)] inhibited both microorganisms while 2 inhibited only S. aureus (11.7 +/- 0.6 mm). Compound 4 did not restrain the growth of any tested microorganism. CONCLUSION: Our results showed that extracts and isolated flavones from Z. tuberculosa may be particularly useful against two pathogenic microorganisms, S. aureus and C. albicans. These results may justify the popular use this species since some fractions tested had antimicrobial activity and others showed significant toxic effects on brine shrimps. However, in order to evaluate possible clinical application in therapy of infectious diseases, further studies about the safety and toxicity of isolated compounds are needed.
Virucidal agents in the eve of manorapid synergy.[Pubmed:20200679]
GMS Krankenhhyg Interdiszip. 2007 Sep 13;2(1):Doc18.
Virucidal agents are chemical substances that attack and inactivate viral particles outside the cell (virions). In general this is accomplished by damaging their protein shells (capsid) or the substance penetrates the core itself, where it destroys the genetic material. Damage to the virion structure is also possible. These agents are used not only for traditional surface disinfection or sterilization of blood, blood products, and other medicinal products as well as in antiviral chemotherapy. They have also been used in recent times for inactivation of viruses in foodstuffs, detergents or cosmetics. Below is given an overview of the data currently available on the performance of these substances when used for the latter applications (cleaning and cosmetics). These include:hydrogen peroxide, hypochlorites, cupric and ferric ions, per-acidsethanol, parachlorometaxylenol in a sodium C14-16 olefin sulfonate, glutaraldehyde, quaternary ammonium salts, chlorhexidine and chlorhexidine gluconate, curdline sulphate, glycerol, lipids, azodicarbonamide, cicloxolone sodium, dichlorisocyanuric acid (sodium salt), benzalkonium salts, disulfate benzamides and benzisothiazolones, congo red, ascorbic acid, nonoxynol-9, para-aminobenzoic acid, bis(monosuccinamide) derivative of p,p'-bis(2-aminoethyl) diphenlyi-C60) (fullerene).merocyanine, benzoporphyrin derivative monoacid ring A, rose bengal, hypericin, hypocrellin A, anthraquinones extracted from plants, sulfonated anthraquinones and other anthraquinone derivativesGRAMICIDINE, GOSSYPOL, GARLIC (ALLIUM SATIVUM) EXTRACT AND ITS COMPONENTS: ajoene, diallyl thiosulfinate (allicin), allyl methyl thioulfinate, methyl allyl thiosulfinate, extracts of ledium, motherworth, celandine, black currant, coaberry and bilberry, extract of Cordia salicifolia, steam distillate from Houttuynia cordata (Saururaceae) and its component, 5,6,7-Trimethoxyflavone from Calicarpa japonica, isoscullarein (5,7,8,4'-tetrahydroxyflavone) from Scutellaria baikalensis and isoscutellarein-8-methylether, alkaloids and phytosteryl ester compounds.
Pyrano chalcones and a flavone from Neoraputia magnifica and their Trypanosoma cruzi glycosomal glyceraldehyde-3-phosphate dehydrogenase-inhibitory activities.[Pubmed:11130676]
Phytochemistry. 2000 Nov;55(6):643-51.
The fruits of Neoraptua magnifica var. magnifica afforded three new flavonoids: 2'-hydroxy-4,4',-dimethoxy-5',6'-(2'',2''-dimethylpyrano)chalcone, 2'-hydroxy-3,4,4'-trimethoxy-5',6'-(2'',2''-dimethylpyrano)chalcone, and 3',4'-methylenedioxy-5,7-dimethoxyflavone which were identified on the basis of spectroscopic methods. The known flavonoids 2'-hydroxy-3,4,4',5-tetramethoxy-5',6'-(2'',2''-dimethylpyrano)chalcone, 2'-hydroxy-3,4,4',5,6'-pentamethoxychalcone, 3',4'-methylenedioxy-5,6,7-Trimethoxyflavone, 3',4'-methylenedioxy-5',5,6,7-tetramethoxyflavone, 3',4',5',5,7-pentamethoxyflavanone and 3',4',5'5,7-pentamethoxyflavone were also identified. The latter flavone was the most active as glyceraldehyde-3-phosphate dehydrogenase-inhibitor.
Antiviral activity of 5,6,7-trimethoxyflavone and its potentiation of the antiherpes activity of acyclovir.[Pubmed:9222055]
J Antimicrob Chemother. 1997 Jun;39(6):821-4.
A naturally occurring flavone, 5,6,7-Trimethoxyflavone (TMF), isolated from the plant Callicarpa japonica, was subjected to antiviral assays. The compound exhibited relatively high inhibitory effects on herpes simplex virus type 1 (HSV-1), human cytomegalovirus and poliovirus. The anti-HSV-1 action was not due to the inhibition of virus adsorption, entry and viral protein synthesis, but might involve, at least in part, a virucidal activity, which results in a suppression of viral binding to host cells at an early replication stage. TMF and acylovir were synergic in their anti-HSV activities at levels below the 50% inhibitory concentrations for antiviral activity.


