alpha-HexylcinnamaldehydeCAS# 101-86-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 101-86-0 | SDF | Download SDF |
| PubChem ID | 1550884 | Appearance | Powder |
| Formula | C15H20O | M.Wt | 216.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
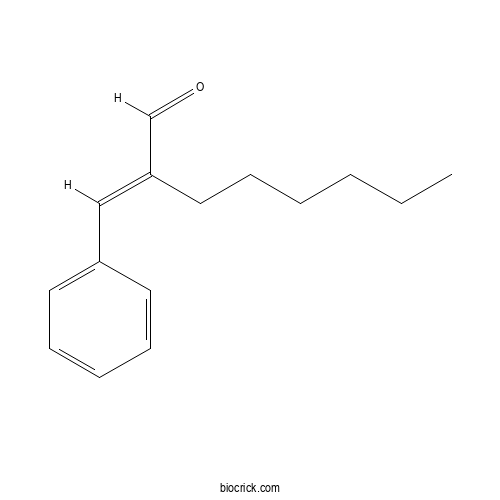
| Chemical Name | (2E)-2-benzylideneoctanal | ||
| SMILES | CCCCCCC(=CC1=CC=CC=C1)C=O | ||
| Standard InChIKey | GUUHFMWKWLOQMM-NTCAYCPXSA-N | ||
| Standard InChI | InChI=1S/C15H20O/c1-2-3-4-6-11-15(13-16)12-14-9-7-5-8-10-14/h5,7-10,12-13H,2-4,6,11H2,1H3/b15-12+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | alpha-Hexylcinnamaldehyde and p-tert-butyl-alpha-methylhydrocinnamic aldehyde are synthetic aldehydes, characterized by a typical floral scent, which makes them suitable to be used as fragrances in personal care (perfumes, creams, shampoos, etc.) and household products, and as flavouring additives in food and pharmaceutical industry. alpha-Hexylcinnamaldehyde is a weak allergen. | |||||

alpha-Hexylcinnamaldehyde Dilution Calculator

alpha-Hexylcinnamaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6232 mL | 23.116 mL | 46.2321 mL | 92.4642 mL | 115.5802 mL |
| 5 mM | 0.9246 mL | 4.6232 mL | 9.2464 mL | 18.4928 mL | 23.116 mL |
| 10 mM | 0.4623 mL | 2.3116 mL | 4.6232 mL | 9.2464 mL | 11.558 mL |
| 50 mM | 0.0925 mL | 0.4623 mL | 0.9246 mL | 1.8493 mL | 2.3116 mL |
| 100 mM | 0.0462 mL | 0.2312 mL | 0.4623 mL | 0.9246 mL | 1.1558 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Calvatic acid
Catalog No.:BCN9778
CAS No.:54723-08-9
- 3,4-Dimethoxyacetophenone
Catalog No.:BCN9777
CAS No.:1131-62-0
- 3,5-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN9776
CAS No.:4319-02-2
- Nortrachelogenin-5'-C-beta-glucoside
Catalog No.:BCN9775
CAS No.:858127-39-6
- Glucofrangulin A
Catalog No.:BCN9774
CAS No.:21133-53-9
- DL-Tyrosine
Catalog No.:BCN9773
CAS No.:556-03-6
- 4'-Methoxychalcone
Catalog No.:BCN9772
CAS No.:959-23-9
- (-)-Eburnamonine
Catalog No.:BCN9771
CAS No.:4880-88-0
- Comanthosid A
Catalog No.:BCN9770
CAS No.:70938-59-9
- 2'-Methoxyflavone
Catalog No.:BCN9769
CAS No.:19725-47-4
- N-trans-caffeoyloctopamine
Catalog No.:BCN9768
CAS No.:1378868-10-0
- Rebaudioside M
Catalog No.:BCN9767
CAS No.:1220616-44-3
- (+/-)-Anabasine
Catalog No.:BCN9780
CAS No.:13078-04-1
- Cannabisin B
Catalog No.:BCN9781
CAS No.:144506-17-2
- Deacylgymnemic acid
Catalog No.:BCN9782
CAS No.:121686-42-8
- Cinobufotenine
Catalog No.:BCN9783
CAS No.:60657-23-0
- Indole
Catalog No.:BCN9784
CAS No.:120-72-9
- 5,6,7-Trimethoxyflavone
Catalog No.:BCN9785
CAS No.:973-67-1
- 3-Methyl-1-butanol
Catalog No.:BCN9786
CAS No.:123-51-3
- Quercetin 3-O-beta-D-glucosyl-(1->2)-rhamnoside
Catalog No.:BCN9787
CAS No.:143016-74-4
- Ethyl phenylacetate
Catalog No.:BCN9788
CAS No.:101-97-3
- Kaempferol 3-robinoside 7-glucoside
Catalog No.:BCN9789
CAS No.:114924-89-9
- Resokaempferol
Catalog No.:BCN9790
CAS No.:2034-65-3
- Ginkgotoxin hydrochloride
Catalog No.:BCN9791
CAS No.:3131-27-9
Safety and efficacy of aryl-substituted primary alcohol, aldehyde, acid, ester and acetal derivatives belonging to chemical group 22 when used as flavourings for all animal species.[Pubmed:32625398]
EFSA J. 2017 Feb 1;15(2):e04672.
Following a request from the European Commission, the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of 18 compounds belonging to chemical group (CG) 22. They are currently authorised as flavours in food. The FEEDAP Panel concludes that: cinnamaldehyde [05.014] is safe at the maximum use level of 125 mg/kg complete feed for salmonids, veal calves and dogs, and at 25 mg/kg for the remaining target species; cinnamyl alcohol [02.017], 3-phenylpropan-1-ol [02.031], 3-(p-cumenyl)-2-methylpropionaldehyde [05.045], alpha-methylcinnamaldehyde [05.050], 3-phenylpropanal [05.080], cinnamic acid [08.022], cinnamyl acetate [09.018], cinnamyl butyrate [09.053], 3-phenylpropyl isobutyrate [09.428], cinnamyl isovalerate [09.459], cinnamyl isobutyrate [09.470], ethyl cinnamate [09.730], methyl cinnamate [09.740] and isopentyl cinnamate [09.742] are safe at the proposed maximum use level of 5 mg/kg complete feed for all target species; 2-phenylpropanal [05.038], alpha-pentylcinnamaldehyde [05.040] and alpha-Hexylcinnamaldehyde [05.041] are safe at the proposed maximum dose level of 5 mg/kg complete feed for all target species except cats, for which 1 mg/kg is safe. No safety concern would arise for the consumer from the use of these compounds up to the highest proposed level in feeds. Irritation and sensitisation hazards for skin and irritation for eye are recognised for the majority of the compounds under application. Respiratory exposure may also be hazardous. For the majority of the compounds belonging to CG 22, the maximum proposed use levels are considered safe for the environment. For alpha-pentylcinnamaldehyde and alpha-Hexylcinnamaldehyde, a use level up to 0.1 mg/kg feed would not cause a risk for the terrestrial and fresh water compartments. Because all the compounds under assessment are used in food as flavourings and their function in feed is essentially the same as that in food, no further demonstration of efficacy is necessary.
Effects of pharmaceuticals and personal care products (PPCPs) on multixenobiotic resistance (MXR) related efflux transporter activity in zebrafish (Danio rerio) embryos.[Pubmed:27810576]
Ecotoxicol Environ Saf. 2017 Feb;136:14-23.
Certain ATP binding cassette (ABC) transporter proteins, such as zebrafish Abcb4, are efflux pumps acting as a cellular defence against a wide range of different, potentially toxic chemical compounds thus mediating so called multixenobiotic resistance (MXR). Certain chemicals target MXR proteins and, as so called chemosensitisers, inhibit the activity of these proteins thus increasing the toxicity of other chemicals that would normally be effluxed. In this study 14 pharmaceuticals and personal care products (PPCPs) that are being increasingly detected in aquatic systems, were assessed for interference with the MXR system of zebrafish (Danio rerio). Concentration dependent effects of test compounds were recorded with the dye accumulation assay using zebrafish embryos and in ATPase assays with recombinant zebrafish Abcb4. In the dye accumulation assay embryos at 24h post fertilisation (hpf) were exposed to 8microm rhodamine 123 along with test compounds for 2h. The rhodamine 123 tissue levels upon the exposure served as a measure for MXR transporter efflux activity of the embryo (low rhodamine levels - high activity; high levels - low activity). The known ABC protein inhibitors MK571, vinblastine and verapamil served as positive controls. All tested PPCPs affected rhodamine 123 accumulation in embryos. For seven compounds rhodamine tissue levels were either both decreased and increased depending on the compound concentration indicating both stimulation and inhibition of rhodamine 123 efflux by those compounds, only increased (inhibition, six compounds) or only decreased (stimulation, one compound). Recombinant zebrafish Abcb4 was obtained with the baculovirus expression system and PPCPs were tested for stimulation/inhibition of basal transporter ATPase activity and for inhibition of the transporter ATPase activity stimulated with verapamil. Eight of the tested PPCPs showed effects on Abcb4 ATPase activity indicating that their effects in the dye accumulation assay may have indeed resulted from interference with Abcb4-mediated rhodamine 123 efflux. Slight stimulatory effects were found for musk xylene, nerol, isoeugenol, alpha-amylcinnamaldehyde, alpha-Hexylcinnamaldehyde and simvastatin indicating Abcb4 substrate/competitive inhibitor properties of those compounds. Likewise, decreases of the verapamil-stimulated Abcb4 ATPase activity by diclofenac and fluoxetine may indicate competitive transporter inhibition. Sertraline inhibited the basal and verapamil-stimulated Abcb4 ATPase activities suggesting its property as non-competitive Abcb4 inhibitor. Taken together, our finding that chemically diverse PPCPs interfere with MXR efflux activity of zebrafish indicates that (1) efflux transporters may influence bioaccumulation of many PPCPs in fish and that (2) many PPCPs may act as chemosensitisers. Furthermore, it appears that interference of PPCPs with efflux activity in zebrafish embryos is not only from effects on Abcb4 but also on other efflux transporter subtypes.
alpha-Hexylcinnamaldehyde Synergistically Increases Doxorubicin Cytotoxicity Towards Human Cancer Cell Lines.[Pubmed:27354593]
Anticancer Res. 2016 Jul;36(7):3347-51.
AIM: alpha-Hexylcinnamaldehyde (HCA), a compound derived from cinnamaldehyde, was evaluated for its potential chemosensitizing properties. MATERIALS AND METHODS: The cytotoxicity of HCA was tested against Caco-2, CCRF/CEM and CEM/ADR5000 human cancer cells. Furthermore, its ability to increase doxorubicin cytotoxicity was evaluated in combination assays. Rhodamine123 efflux assay was carried out in order to highlight the possible interference of HCA with functionality of ATP-binding cassette (ABC)-transporters. RESULTS: In spite of a low cytotoxicity, HCA increased the antiproliferative effect of doxorubicin in all the cell lines tested, being particularly effective in CCRF/CEM. The compound also reduced the rhodamine123 efflux in Caco-2 and CEM/ADR5000 cells, suggesting a possible interference with ABC transporter functionality. CONCLUSION: Considering that the greatest synergism between HCA and DOX was found against CCRF/CEM cells (lacking ABC pumps), it seems likely that non-specific mechanisms, including the alteration of membrane permeability, could be involved in the chemosensitizing effect of HCA.
Interaction of alpha-Hexylcinnamaldehyde with a Biomembrane Model: A Possible MDR Reversal Mechanism.[Pubmed:25893313]
J Nat Prod. 2015 May 22;78(5):1154-9.
The ability of the naturally derived compound alpha-Hexylcinnamaldehyde (1) to interact with biomembranes and to modulate their permeability has been investigated as a strategy to reverse multidrug resistance (MDR) in cancer cells. Dimyristoylphosphatidylcholine (DMPC) multilamellar vesicles (MLVs) were used as biomembrane models, and differential scanning calorimetry was applied to measure the effect of 1 on the thermotropic behavior of DMPC MLVs. The effect of an aqueous medium or a lipid carrier on the uptake of 1 by the biomembrane was also characterized. Furthermore, taking into account that MDR is strictly regulated by redox signaling, the pro-oxidant and/or antioxidant effects of 1 were evaluated by the crocin-bleaching assay, in both hydrophilic and lipophilic environments. Compound 1 was uniformly distributed in the phospholipid bilayers and deeply interacted with DMPC MLVs, intercalating among the phospholipid acyl chains and thus decreasing their cooperativity. The lipophilic medium allowed the absorption of 1 into the phospholipid membrane. In the crocin-bleaching assay, the substance produced no pro-oxidant effects in both hydrophilic and lipophilic environments; conversely, a significant inhibition of AAPH-induced oxidation was exerted in hydrophilic medium. These results suggest a possible role of 1 as a chemopreventive and chemosensitizing agent for fighting cancer.
alpha-Hexylcinnamaldehyde inhibits the genotoxicity of environmental pollutants in the bacterial reverse mutation assay.[Pubmed:25494477]
J Nat Prod. 2014 Dec 26;77(12):2664-70.
The antimutagenicity of alpha-Hexylcinnamaldehyde (1), a semisynthetic and more stable derivative of cinnamaldehyde, was evaluated against common environmental pollutants in the bacterial reverse mutation assay. The pre-, co-, and post-treatment protocols were applied to assess the involvement of desmutagenic and/or bioantimutagenic mechanisms. Compound 1 (9-900 muM) produced a strong antimutagenicity (>40% inhibition) in the Salmonella typhimurium TA98 strain against the nitroarenes 2-nitrofluorene and 1-nitropyrene in almost all experimental conditions. A strong inhibition was also reached against the nitroarene 1,8-dinitropyrene and the arylamine 2-aminoanthracene in the cotreatment at the highest concentrations tested. In order to evaluate if an inhibition of bacterial nitroreductase (NR) and O-acetyltransferase (OAT) could be involved in the antimutagenicity of 1 against nitroarenes, the substance was further tested against 1-nitropyrene (activated by both NR and OAT) in TA98NR and TA98 1,8-DNP strains (lacking the NR and OAT enzymes, respectively). Although both desmutagenic and bioantimutagenic mechanisms appear mostly involved in the antimutagenicity of 1, based on data obtained in the TA98NR strain, applying the pretreatment protocol, compound 1 seems to act as an inhibitor of the OAT-mediated mutagen bioactivation. These results provide justification for further studies on 1 as a possible chemopreventive agent.
Genotoxicity assessment of some cosmetic and food additives.[Pubmed:24239523]
Regul Toxicol Pharmacol. 2014 Feb;68(1):16-22.
alpha-Hexylcinnamaldehyde (HCA) and p-tert-butyl-alpha-methylhydrocinnamic aldehyde (BMHCA) are synthetic aldehydes, characterized by a typical floral scent, which makes them suitable to be used as fragrances in personal care (perfumes, creams, shampoos, etc.) and household products, and as flavouring additives in food and pharmaceutical industry. The aldehydic structure suggests the need for a safety assessment for these compounds. Here, HCA and BMHCA were evaluated for their potential genotoxic risk, both at gene level (frameshift or base-substitution mutations) by the bacterial reverse mutation assay (Ames test), and at chromosomal level (clastogenicity and aneuploidy) by the micronucleus test. In order to evaluate a primary and repairable DNA damage, the comet assay has been also included. In spite of their potential hazardous chemical structure, a lack of mutagenicity was observed for both compounds in all bacterial strains tested, also in presence of the exogenous metabolic activator, showing that no genotoxic derivatives were produced by CYP450-mediated biotransformations. Neither genotoxicity at chromosomal level (i.e. clastogenicity or aneuploidy) nor single-strand breaks were observed. These findings will be useful in further assessing the safety of HCA and BMHCA as either flavour or fragrance chemicals.
[Skin irritation and sensitization of swine acellular dermal matrix treated with hyaluronic acid].[Pubmed:23290759]
Zhonghua Shao Shang Za Zhi. 2012 Oct;28(5):344-8.
OBJECTIVE: To evaluate the skin irritation and sensitization potential of the swine acellular dermal matrix treated with hyaluronic acid (SADM-HA). METHODS: (1) Skin irritation test. Twelve New Zealand rabbits were divided into SADM-HA group, allogeneic skin group, and (human) xeno-skin group according to the random number table, with 4 rabbits in each group. Four test sites were designed on the back of each rabbit. Two test sites of each rabbit in the three groups were covered with SADM-HA, allogeneic skin, and xeno-skin, respectively. Another test site was covered with gauze containing 200 g/L sodium dodecyl sulfate solution as positive control. The last test site was covered with gauze containing normal saline as negative control. The primary irritation index and cumulative irritation index of each material were calculated. (2) Skin closed-patch test. Sixty guinea pigs were used. Fifty-four guinea pigs were divided into SADM-HA group, allogeneic skin group, and (human) xeno-skin group according to the random number table, with 18 guinea pigs in each group. Twelve guinea pigs in each of the three groups were correspondingly induced and stimulated by SADM-HA, allogeneic skin, and xeno-skin, with 6 guinea pigs in each group treated with ethanol-soaked gauze to serve as negative control. The remaining 6 guinea pigs were treated with gauze containing 25% alpha-Hexylcinnamaldehyde ethanol solution as positive control. The rating scales of Magnusson and Kligman were used to grade the condition of skin after being treated with above-mentioned materials to evaluate skin sensitivity to them at post stimulation hour 24 and 48. Data were processed with the non-parametric test of independent samples. RESULTS: (1) In the skin irritation test, the primary irritation indexes of the three dressings in SADM-HA group, allogeneic skin group, and xeno-skin group were respectively -0.04, 0.13, and 0.08. The cumulative irritation indexes of the three dressings in SADM-HA group, allogeneic skin group, and xeno-skin group were respectively 0.27, 0.10, and 0.25, which were close to those of negative control within the three groups. The skin irritation of each of the three materials was negligible. (2) In the skin closed-patch test, all scores of the three dressings in SADM-HA group, allogeneic skin group, and xeno-skin group were between 0 and 1. The scores of SADM-HA group and allogeneic skin group were close to those of negative control within the two groups (with U values respectively 188.00 and 90.00, P values both above 0.05). The differences were statistically significant between each material of the three groups and positive control (with U values respectively 19.00, 59.00, 21.50, P values all below 0.01). CONCLUSIONS: The SADM-HA is safe and reliable without skin irritation and sensitization, and it has encouraging prospect in clinical application.
Predicting skin sensitization potential and inter-laboratory reproducibility of a human Cell Line Activation Test (h-CLAT) in the European Cosmetics Association (COLIPA) ring trials.[Pubmed:20510347]
Toxicol In Vitro. 2010 Sep;24(6):1810-20.
Regulatory policies in Europe prohibited the testing of cosmetic ingredients in animals for a number of toxicological endpoints. Currently no validated non-animal test methods exist for skin sensitization. Evaluation of changes in cell surface marker expression in dendritic cell (DC)-surrogate cell lines represents one non-animal approach. The human Cell Line Activation Test (h-CLAT) examines the level of CD86 and CD54 expression on the surface of THP-1 cells, a human monocytic leukemia cell line, following 24h of chemical exposure. To examine protocol transferability, between-lab reproducibility, and predictive capacity, the h-CLAT has been evaluated by five independent laboratories in several ring trials (RTs) coordinated by the European Cosmetics Association (COLIPA). The results of the first and second RTs demonstrated that the protocol was transferable and basically had good between-lab reproducibility and predictivity, but there were some false negative data. To improve performance, protocol and prediction model were modified. Using the modified prediction model in the first and second RT, accuracy was improved. However, about 15% of the outcomes were not correctly identified, which exposes some of the limitations of the assay. For the chemicals evaluated, the limitation may due to chemical being a weak allergen or having low solubility (ex. alpha-Hexylcinnamaldehyde). The third RT evaluated the modified prediction model and satisfactory results were obtained. From the RT data, the feasibility of utilizing cell lines as surrogate DC in development of in vitro skin sensitization methods shows promise. The data also support initiating formal pre-validation of the h-CLAT in order to fully understand the capabilities and limitations of the assay.
Effects of strain differences and vehicles on results of local lymph node assays.[Pubmed:20484859]
Exp Anim. 2010;59(2):245-9.
The Local Lymph Node Assay (LLNA) is now regarded as the worldwide standard. The analysis of accumulated LLNA data reveals that the animal strains and vehicles employed are likely to affect LLNA results. Here we show that an obvious strain difference in the local lymph node response was observed between DMSO-treated CBA/CaOlaHsd and CBA/CaHsdRcc mice. We also show that a vehicle difference in the response was observed when CBA/CaHsdRcc mice were exposed to 6 vehicles; 4:1 v/v acetone/olive oil (AOO), ethanol/water (70% EtOH), N,N-dimethylformamide (DMF), 2-butanone (BN), propylene glycol (PG), and dimethylsulfoxide (DMSO). The dpm/LN level was lowest in the 70% EtOH group and highest in the DMSO group. When alpha-Hexylcinnamaldehyde (HCA) was used as a sensitizer for the LLNA, HCA was a weak sensitizer when AOO or DMSO was used as a vehicle, but a moderate sensitizer when the other 4 vehicles were used. This study showed that there are vehicle differences in the local lymph node response (dpm/LN level) in the LLNA and that the sensitization potency of HCA may be classified in different categories when using different vehicles. This suggests that careful consideration should be exercised in selecting a vehicle for the LLNA. A further comprehensive study will be needed to investigate why vehicle differences are observed in the LLNA.
Quantitative determination of some volatile suspected allergens in cosmetic creams spread on skin by direct contact sorptive tape extraction-gas chromatography-mass spectrometry.[Pubmed:20074740]
J Chromatogr A. 2010 Apr 16;1217(16):2599-605.
This study describes a method based on direct contact sorptive tape extraction followed by on-line thermal desorption gas chromatography-mass spectrometry (DC-STE-GC-MS) to detect and quantify a group of suspected volatile allergens on the European Union (E.U.) list and a related compound on the skin (the stratum corneum) of volunteers treated with a cream of known composition fortified with the reference allergens. The following compounds were tested: citronellol, Z-citral (neral), geraniol, cinnamaldehyde, anisyl alcohol, cinnamyl alcohol, eugenol, methyleugenol, coumarin, isoeugenol, alpha-isomethylionone, 2-(4-tert-butylbenzyl)propionaldehyde (lilial), alpha-amylcinnamaldehyde, alpha-Hexylcinnamaldehyde. Sorptive tape extraction (STE) is a sorption-based sampling technique in which a flexible polydimethylsiloxane (PDMS) tape is used to recover analytes by direct contact with the surface of a solid matrix or from the headspace in equilibrium with it. The reliability of the method was confirmed by: (i) allergen recoveries varying from 52.3% for lilial to 95.7% for neral, (ii) linearity in the range 10-150ppm, with regression coefficient R(2) always above 0.97, (iii) repeatability of each analyte, RSD% never exceeding 10%, (iv) intermediate precision, always below 15%, and (v) LOD and LOQ in the ppb range, therefore fully compatible with E.U. prescriptions (ppm). Other parameters such as substantivity analyte, approximate permeation through skin and influence of different nature of stratum corneum on recovery were also investigated. The method was also successfully applied to five commercially available creams declared to contain some of the allergens in question spread on the skin of the same volunteers.
A comparative study of the sensitivity of the 3-induction and 9-induction Buehler test procedures for assessing skin sensitisation potential.[Pubmed:15582197]
Food Chem Toxicol. 2005 Jan;43(1):65-75.
Assessment of skin sensitization potential is a mandatory requirement for the registration or notification of most types of chemicals and products. Until recently, two methods using the guinea pig as test model were the most widely accepted; the guinea pig maximisation test and the Buehler test. In the case of agrochemical formulations, which constitute the final end use product in contact with operators, industry and also some regulatory authorities consider the Buehler method more appropriate as the methodology is more relevant to likely exposure in the field. However, certain European regulatory authorities have become concerned about the sensitivity of the Buehler test for this purpose and have requested that a modified method is used in which additional applications of test materials are used during the induction phase of the protocol (a total of 9 rather than the normal 3). This study was designed to assess whether this modification was justified. Six reference substances (formaldehyde, alpha-Hexylcinnamaldehyde, fragrance mix, thimerosal, mercaptobenzothiazole and phthalic anhydride); all mild to moderate skin sensitizing chemicals, were assessed in a study, which compared the use of 3 and 9 induction applications. The results of this study demonstrated that, although most of these sensitisers were detected by both protocols, the modified method (9 induction applications) was no more sensitive than the standard method (3 induction applications). As the modified protocol is also potentially more stressful to the animals, it is concluded that the use of additional induction applications in the Buehler test cannot be justified from either a scientific or an animal welfare perspective.
Evaluation of lymphocyte subpopulations in draining lymph node cells following allergen and irritant.[Pubmed:21782719]
Environ Toxicol Pharmacol. 2004 Jun;17(2):95-102.
The murine local lymph node assay (LLNA) has been developed as an alternative to guinea pig models for the assessment of the contact sensitization potential. However, there is a need to develop a non-radioisotopic endpoint for the LLNA, because of the radioisotopic method's requiring the use of special facilities. In this study, we investigated to evaluate the lymphocyte subpopulations in the lymph node cells following allergen and irritant treatment. Female Balb/c mice were treated by the topical application on the dorsum of both ears with sensitizers, 2,4-dinitrochlorobenzene (DNCB), toluene diisocyanate (TDI), and alpha-Hexylcinnamaldehyde (HCA), and an irritant, sodium lauryl sulfate (SLS), once daily for three consecutive days. The lymph node (LN) cells were harvested 72h after the final treatment. Phenotypic analysis of lymphocytes subsets was performed with a flow cytometry. The allergens DNCB, TDI, and HCA and an irritant, SLS increased cell number compared to the vehicle. Mice were treated with DNCB, HCA, and TDI showed a preferential increase in the percentage of B220+CD40+ cells compared with vehicle and irritant-treated mice. There was an increase in B220+CD86+ cells of mice treated with DNCB, TDI, and HCA, but no significant increases were observed in mice treated with SLS. Mice were treated with DNCB and TDI showed an increase in the percentage of B220+CD23+ cells compared with vehicle and irritant-treated mice. These results suggest that analysis of B cell activation marker, CD40 on B cells may be useful in differentiating allergen and irritant responses in the draining lymph nodes of chemically treated mice.
Evaluation of cell proliferation in ear and lymph node using BrdU immunohistochemistry for mouse ear swelling test.[Pubmed:21782663]
Environ Toxicol Pharmacol. 2003 Jun;14(1-2):61-8.
The mouse ear swelling test (MEST) was developed as an alternative to guinea pig models for measuring the contact sensitization potential. However, the MEST relies on the quantitative measurement of ear swelling by micrometer as the means of determining the endpoint. The purpose of this study was to investigate the possibility of using cell proliferation in the ear and lymph node by bromodeoxyuridine (BrdU) immunohistochemistry as a reliable marker for MEST. Female Balb/c mice were treated by the topical application of various sensitizers, 2,4-dinitrochlorobenzene (DNCB), toluene diisocyanate (TDI) and alpha-Hexylcinnamaldehyde (HCA) and an irritant, sodium lauryl sulfate (SLS) following the protocol of MEST. The proliferation of cells in the ear and auricular lymph node was analyzed by BrdU incorporations into cells. There were significant increases in the cell proliferations of the ear and auricular lymph node in mice treated with DNCB and TDI compared to the vehicle control. All allergens and the irritant were correctly identified by the MEST using BrdU immunohistochemistry of lymph node responses. The standard MEST assay showed positive results in the case of the strong sensitizers, DNCB and TDI. However, HCA and SLS were not correctly identified in the ear swelling assay. These results suggest that the measurement of cell proliferation in the auricular lymph node using BrdU immunohistochemistry could provide a reliable marker for MEST.
Use of a B cell marker (B220) to discriminate between allergens and irritants in the local lymph node assay.[Pubmed:12151637]
Toxicol Sci. 2002 Aug;68(2):420-8.
It has been shown that exposure of mice to contact allergens induces B cell activation in the draining lymph nodes (DLN), as seen by an increase in the percentage of B220+ or IgG/IgM+ cells. We have now examined whether the measurement of the percentage of B220+ cells could be used as an alternative or supplementary endpoint for the local lymph node assay (LLNA) to differentiate between allergenic responses and those few irritants that induce low-level proliferation in the DLN. Mice were treated on the ears, daily for 3 consecutive days, with various allergens (1-chloro-2,4-dinitrobenzene, alpha-Hexylcinnamaldehyde, trinitrochlorobenzene, isoeugenol, and eugenol) or irritants (benzalkonium chloride, methyl salicylate, salicylic acid, and sodium lauryl sulfate). The DLN were excised 72 h following the final topical treatment, and the cells were prepared for B220 analysis using flow cytometry. The percentage of B220+ cells in lymph nodes derived from test and vehicle-treated animals was determined for 5 allergens and 4 irritants tested in multiple experiments (n = 3 to 17). As expected, the percentage of B220+ B cells was increased with each of the allergens tested, whereas irritant treatment did not cause similar increases. Moreover, the method was reproducible. For example, the strong allergen, 1-chloro-2,4-dinitrobenzene and the weak allergen, alpha-Hexylcinnamaldehyde were identified as allergens in 17 of 17 and in 12 of 13 experiments, respectively. The percentage of B220 values for each chemical treatment (41 observations for allergens; 28 observations for irritants) versus the percentage of B220 values for the concurrent vehicle controls were plotted, and a classification tree model was developed that defined a B220 test:vehicle ratio cutoff of 1.25 for discriminating between allergens (>1.25) and irritants (<1.25). Using this B220 test:vehicle ratio of 1.25 in 93% of the 69 independent observations made, the allergens and irritants tested were identified correctly. Finally, to evaluate the performance of this model in a second independent laboratory, 3 allergens and 2 irritants were tested. Each of the allergens and irritants were classified correctly using the B220 test:vehicle ratio cutoff of 1.25. These data demonstrate that analysis of B220 expression in DLN may be useful in differentiating between allergen and irritant responses induced in chemically treated mice.
Phenotypic alterations and IL-1beta production in CD34(+) progenitor- and monocyte-derived dendritic cells after exposure to allergens: a comparative analysis.[Pubmed:12029496]
Arch Dermatol Res. 2002 May;294(3):109-16.
Dendritic cells (DC) have been shown to capture and process antigens and play an initiating role in contact sensitization. Cells with dendritic morphology can be generated in vitro either from CD34(+) cord blood cells or from CD14(+) peripheral monocytes. The aim of this study was to determine the state of maturation/activation of both populations after exposure to several concentrations of four well-established model allergens (nickel sulfate, eugenol, alpha-Hexylcinnamaldehyde and 2,4,6-trinitrobenzene sulfonic acid) or the irritant sodium dodecyl sulfate. We analyzed the surface expression of CD86, CD83 and HLA-DR and the production of IL-1beta. DC from the two sources were generated separately in two laboratories, but challenged using identical test protocols. Using both DC populations it was possible to detect the allergens under investigation, though minor differences regarding effective concentrations were noted. The non-responsiveness of CD34-DC to CIN was probably due to non-optimal concentrations. Ni(2+), known as a moderate allergen in vivo, showed the most prominent effect in both cell systems. CD86 expression was the most reliable phenotypic marker for the in vitro identification of allergens. Due to substantial individual variations it was difficult to draw any definite conclusions as to the relevance of IL-1beta production as an activation endpoint. We conclude that both test systems are able to respond to allergens, but CD34-DC must be exposed to higher concentrations to demonstrate significant phenotypic changes. On the other hand, Mo-DC from only some of the donors reacted to allergens, in contrast to CD34-DC, which responded to allergens irrespective of the donor, thus necessitating the use of Mo-DC cultures from several blood donors.


