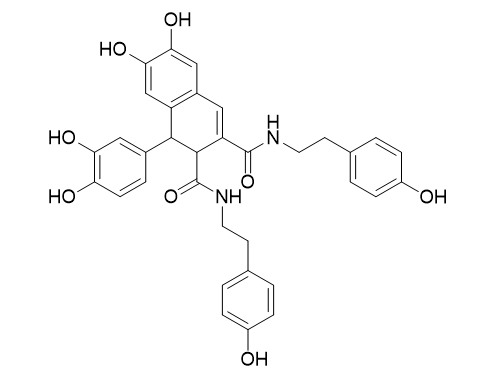
Cannabisin BCAS# 144506-17-2 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 144506-17-2 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C34H32N2O8 | M.Wt | 596.6 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cannabisin B possesses considerable antiproliferative activity and that it may be utilised as a promising chemopreventive agent against hepatoblastoma disease. It induces autophagic cell death by inhibiting the AKT/mTOR pathway and S phase cell cycle arrest in HepG2 cell. | |||||

Cannabisin B Dilution Calculator

Cannabisin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6762 mL | 8.3808 mL | 16.7616 mL | 33.5233 mL | 41.9041 mL |
| 5 mM | 0.3352 mL | 1.6762 mL | 3.3523 mL | 6.7047 mL | 8.3808 mL |
| 10 mM | 0.1676 mL | 0.8381 mL | 1.6762 mL | 3.3523 mL | 4.1904 mL |
| 50 mM | 0.0335 mL | 0.1676 mL | 0.3352 mL | 0.6705 mL | 0.8381 mL |
| 100 mM | 0.0168 mL | 0.0838 mL | 0.1676 mL | 0.3352 mL | 0.419 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+/-)-Anabasine
Catalog No.:BCN9780
CAS No.:13078-04-1
- alpha-Hexylcinnamaldehyde
Catalog No.:BCN9779
CAS No.:101-86-0
- Calvatic acid
Catalog No.:BCN9778
CAS No.:54723-08-9
- 3,4-Dimethoxyacetophenone
Catalog No.:BCN9777
CAS No.:1131-62-0
- 3,5-Dihydroxy-4-methoxybenzoic acid
Catalog No.:BCN9776
CAS No.:4319-02-2
- Nortrachelogenin-5'-C-beta-glucoside
Catalog No.:BCN9775
CAS No.:858127-39-6
- Glucofrangulin A
Catalog No.:BCN9774
CAS No.:21133-53-9
- DL-Tyrosine
Catalog No.:BCN9773
CAS No.:556-03-6
- 4'-Methoxychalcone
Catalog No.:BCN9772
CAS No.:959-23-9
- (-)-Eburnamonine
Catalog No.:BCN9771
CAS No.:4880-88-0
- Comanthosid A
Catalog No.:BCN9770
CAS No.:70938-59-9
- 2'-Methoxyflavone
Catalog No.:BCN9769
CAS No.:19725-47-4
- Deacylgymnemic acid
Catalog No.:BCN9782
CAS No.:121686-42-8
- Cinobufotenine
Catalog No.:BCN9783
CAS No.:60657-23-0
- Indole
Catalog No.:BCN9784
CAS No.:120-72-9
- 5,6,7-Trimethoxyflavone
Catalog No.:BCN9785
CAS No.:973-67-1
- 3-Methyl-1-butanol
Catalog No.:BCN9786
CAS No.:123-51-3
- Quercetin 3-O-beta-D-glucosyl-(1->2)-rhamnoside
Catalog No.:BCN9787
CAS No.:143016-74-4
- Ethyl phenylacetate
Catalog No.:BCN9788
CAS No.:101-97-3
- Kaempferol 3-robinoside 7-glucoside
Catalog No.:BCN9789
CAS No.:114924-89-9
- Resokaempferol
Catalog No.:BCN9790
CAS No.:2034-65-3
- Ginkgotoxin hydrochloride
Catalog No.:BCN9791
CAS No.:3131-27-9
- Isoamyl butyrate
Catalog No.:BCN9792
CAS No.:106-27-4
- 4',5,7-Trihydroxy 3,6,8-trimethoxyflavone
Catalog No.:BCN9793
CAS No.:57393-71-2
Phenolics, acyl galactopyranosyl glycerol, and lignan amides from Tetragonia tetragonioides (Pall.) Kuntze.[Pubmed:30263405]
Food Sci Biotechnol. 2016 Oct 31;25(5):1275-1281.
Eleven antioxidative compounds, including five lignin amides, were isolated from the aerial part of Tetragonia tetragonioides (New Zealand spinach) using 1,1-diphenyl-2-picrylhydrazyl radicalscavenging assay-guided purification. The structures were determined by nuclear magnetic resonance and electrospray ionization-mass spectroscopy. These compounds were identified as methyl linoleate (1), methyl coumarate (2), methyl ferulate (3), 1-O-stearoyl-3-O-beta-D-galactopyranosyl-sn-glycerol (4), 1-O-caffeoyl-beta-D-glucopyranoside (5), N-trans-caffeoyltyramine (6), Cannabisin B (7), cannabisin A (8), Ntrans-feruloyltyramine (9), N-cis-feruloyltyramine (10), and N-trans-sinapoyltyramine (11). Compounds 1, 2, 4, 5, and 8-11 were isolated for the first time from this plant.
Characterization of byproducts originating from hemp oil processing.[Pubmed:25426777]
J Agric Food Chem. 2014 Dec 24;62(51):12436-42.
Valorization of hemp seed meal, a byproduct of hemp oil processing, was performed by measuring the distribution of nutritional and antinutritional compounds in different hemp seed meal fractions. According to chemical composition, two cotyledon-containing fractions (>180 and <180 mum) were significantly richer in protein (p < 0.05) (41.2% +/- 0.04% and 44.4% +/- 0.02%, respectively), lipid (15.1% +/- 0.02% and 18.6% +/- 0.04%, respectively), and sugar content (4.96% +/- 0.11% and 3.46% +/- 0.08%, respectively) in comparison to the hull-containing fractions (>350 and >250 mum), which were significantly richer in crude fiber content (29.5% +/- 0.04% and 21.3% +/- 0.03%, respectively). The free radical scavenging capacity (IC50) of fraction extracts increased (p < 0.05) with increasing mean particle size (from 17.18 +/- 0.59 to 5.29 +/- 0.30 mg/mL). Cannabisin B and N-trans-caffeoyltyramine were the most abundant phenolic compounds in the hull fractions (from 267 +/- 15.9 to 287 +/- 23.1 mg/kg), while cotyledon fractions had higher content of catechin (from 313 +/- 12.4 to 744 +/- 22.2 mg/kg) and p-hydroxybenzoic acid (from 124 +/- 6.47 to 129 +/- 8.56 mg/kg (P < 0.05). Well-balanced omega-6 to omega-3 fatty acid ratio (3:1) was determined in all fractions. Antinutrients (trypsin inhibitors, phytic acid, glucosinolates, and condensed tannins) were mostly located in the cotyledon fractions. These findings indicate that the separation of hemp seed meal into different fractions could be used to concentrate valuable target compounds and consequently facilitate their recovery.
Cannabisin B induces autophagic cell death by inhibiting the AKT/mTOR pathway and S phase cell cycle arrest in HepG2 cells.[Pubmed:23411211]
Food Chem. 2013 Jun 1;138(2-3):1034-41.
This study investigates the anticancer properties of Cannabisin B, purified from hempseed hull, in HepG2 human hepatoblastoma cells. The results indicate that Cannabisin B significantly inhibited cell proliferation by inducing autophagic cell death rather than typical apoptosis. Cell viability transiently increased upon the addition of a low concentration of Cannabisin B but decreased upon the addition of high concentrations. Cannabisin B-induced changes in cell viability were completely inhibited by pre-treatment with 3-methyladenine (3-MA), indicating that the induction of autophagy by Cannabisin B caused cell death. Additionally, Cannabisin B induced S phase cell cycle arrest in a dose-dependent manner. Moreover, Cannabisin B was found to inhibit survival signaling by blocking the activation of AKT and down-stream targets of the mammalian target of rapamycin (mTOR). These findings suggest that Cannabisin B possesses considerable antiproliferative activity and that it may be utilised as a promising chemopreventive agent against hepatoblastoma disease.
The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.).[Pubmed:23107724]
Food Chem. 2012 Sep 15;134(2):1030-7.
Forty samples were extracted from defatted kernels and hulls of two varieties of hempseed (Bama and Yunma No. 1) using 10 different polar solvent systems. The radical scavenging capacity of the extracts was evaluated using 2,2-diphenyl-1-pikrylhydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assays and the total phenolic content was determined by Folin-Ciocalteu's phenol reagent. The correlation analysis indicated that the antioxidants in hempseed belonged to phenolic and DPPH() assay was suitable for evaluating the radical scavenging activity. Two compounds, with predominant antiradical activity, were isolated in 60% ethanol extract of hempseed hull using macroporous resin absorption, LH-20 gel chromatography, and high performance liquid chromatography methods, which were identified as N-trans-caffeoyltyramine and Cannabisin B by high-resolution mass spectra, nuclear magnetic resonance spectra, and ultraviolet data. The two compounds exhibited significant high DPPH() scavenging activity and protective effect against in vitro oxidation of human low-density lipoprotein compared with extracts from flaxseed, grape seed, and soybean. This suggests that hempseed hull extract is a potential source of natural antioxidants, which could be added to dietary supplements to help prevent oxidative stress.
Cinnamoylphenethyl amides from Polygonum hyrcanicum possess anti-trypanosomal activity.[Pubmed:22816300]
Nat Prod Commun. 2012 Jun;7(6):753-5.
A methanolic extract from aerial parts of Polygonum hyrcanicum (Polygonaceae) showed high activity against Trypanosoma brucei rhodesiense (IC50 = 3.7 microg/mL). Bioassay-guided fractionation of the extract resulted in isolation of cinnamoylphenethyl amides, including N-trans-caffeoyltyramine (1), N-trans-p-coumaroyltyramine (7), and N-trans-feruloyltyramine (8) as the main active constituents (IC50s ranging from 2.2 to 13.3 microM). Some structurally related, but less active compounds, such as Cannabisin B (2), tyrosol (3), p-coumaric acid (4), ferulic acid (5), and N-cis-feruloyltyramine (6) were also identified, along with N-trans-3,4-dimethoxycinnamoyldopamine (9). Cytotoxicity of the active compounds in L6 cells was determined, and selectivity indices (SI) of 7.9 to 33.4 were calculated.


