Chelidonium majus
Chelidonium majus
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
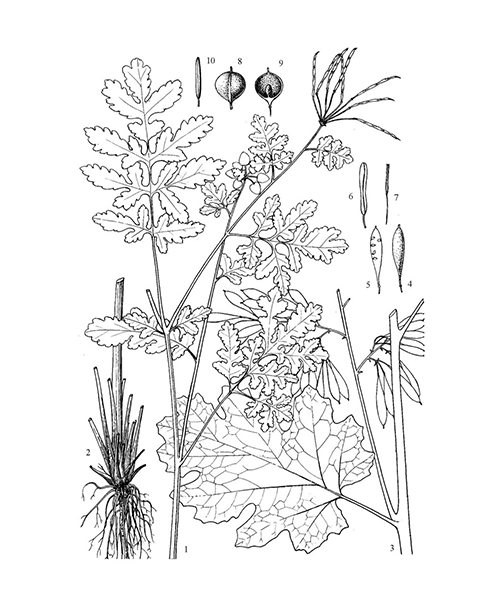
Natural products/compounds from Chelidonium majus
- Cat.No. Product Name CAS Number COA
-
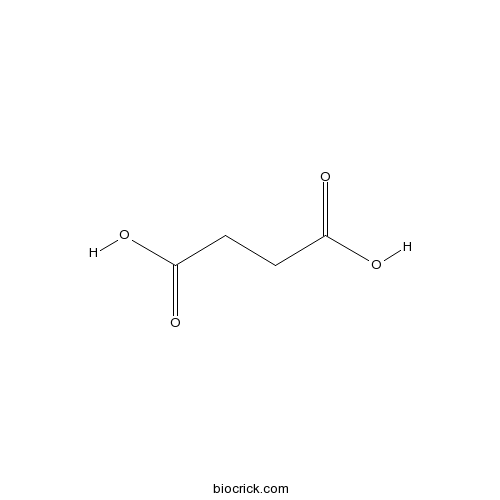
BCN5890
Succinic acid110-15-6
Instructions
-
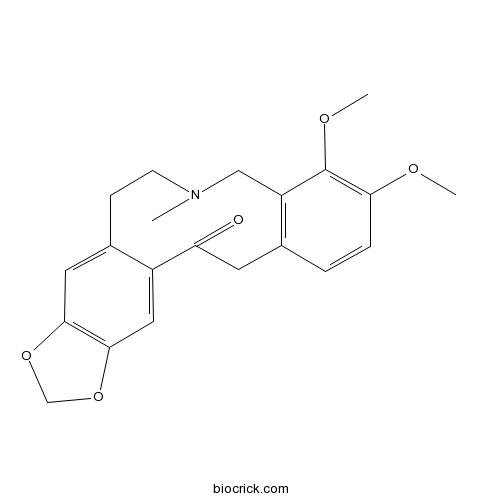
BCN8346
6- Methoxydihydrosanguinarine151890-26-5
Instructions

-
BCN8322
Chelerythrine chloride3895-92-9
Instructions

-
BCN2463
Chelidonine476-32-4
Instructions

-
BCN9043
Allocryptopine485-91-6
Instructions
-
BCC6481
Sanguinarine chloride5578-73-4
Instructions

-
BCN6321
Coptisine chloride6020-18-4
Instructions

-
BCN2273
Dihydrochelerythrine6880-91-7
Instructions

-
BCN5374
Citric acid77-92-9
Instructions
-
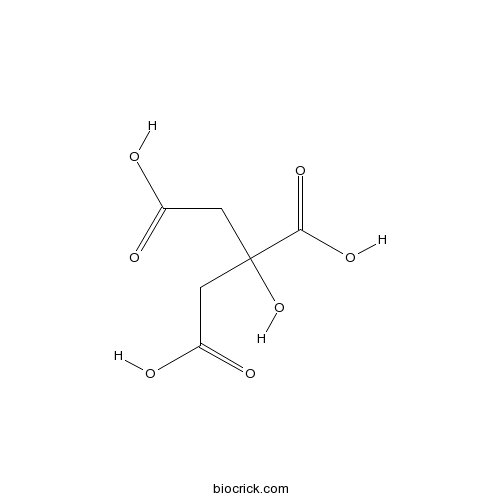
BCN6547
Chelidonic acid99-32-1
Instructions

Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries.[Pubmed: 29908576]
The geographic and ecologic specificity of Romania and other Eastern European countries has resulted in the development of an exceptional diversity of medicinal plants. The purpose of this study was to provide an overview of the ethnobotanical dermatology practices based on the use of medicinal plants in this region. The indications, ethnopharmacologic activities, parts used, and administration of 106 medicinal plants are provided. We also discuss the relative importance of these species, using two modified indices of quantitative ethnobotany: Use Value Index and Relative Dermatologic Importance, which were calculated on the basis of etic constructions (indications and ethnopharmacologic activities). The species identified to have the highest dermatologic importance (on a scale of 100) were Brassica oleracea L. (100), Matricaria chamomilla L. (79.17), Arctium lappa L. (74.82), Daucus carota L. (72.28), Equisetum arvense L. (70.47), Juglans regia L. (69.93), Populous nigra L. (65.94), Symphytum officinale L. (63.59), Chelidonium majus L. (57.78), Calendula officinalis L. (57.78), Achillea millefolium L. (57.43), Melilotus officinalis L. (55.25), Allium cepa L. (51.45), Quercus robur L. (51.08), and Betula spp. (50.91). This preliminary study on ethnobotanical dermatology practices indicates that Eastern European traditional medical knowledge represents an important heritage that is currently underexploited.
Impact of drought and salt stress on the biosynthesis of alkaloids in Chelidonium majus L.[Pubmed: 29783187]
When plants are exposed to various stress situations, their alkaloid concentration frequently is enhanced. This well-known phenomenon is presumably due to a passively enhanced rate of biosynthesis, caused by greatly elevated concentrations of NADPH in stressed plants. Here, we used Chelidonium majus L. plants, which accumulate high concentrations of dihydrocoptisine in their leaves, to study the impact of drought and salt stress on the biosynthesis and accumulation of alkaloids. In comparison to well-watered controls, in the transcriptome of the gene encoding the key enzyme in alkaloid biosynthesis, stylopine synthase, is enhanced in stressed C. majus plants. If we presuppose that increased transcript levels correlate with increased enzymatic activity of the gene products, these data indicate, for the first time, that stress-related increases in alkaloid concentration might not only be caused by the well-known stress-related passive shift, but may also be due to an enhancement of enzymatic capacity.
Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract.[Pubmed: 29177803]
The basic goal of this study was to synthesize zinc oxide nanoparticles using the Chelidonium majus extract and asses their cytotoxic and antimicrobial properties. The synthesized ZnO NPs were characterized by UV-Vis, Scanning Electron Microscopy (SEM) with EDS profile, Fourier Transform Infrared Spectroscopy (FTIR), X-ray diffraction (XRD), Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM). The aforementioned methods confirmed that the size of synthesized ZnO nanoparticles was at the range of 10 nm. The antimicrobial activity of ZnO nanoparticles synthesized using the Ch. majus extract was tested against standard strains of bacteria (Staphylococcus aureus NCTC 4163, Pseudomonas aeruginosa NCTC 6749, Escherichia coli ATCC 25922), yeast (Candida albicans ATCC 10231), filamentous fungi (molds: Aspergillus niger ATCC 16404, dermatophytes: Trichophyton rubrum ATCC 28188), clinical strains of bacteria (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus) and yeast (Candida albicans). The study showed that zinc oxide nanoparticles were excellent antimicrobial agents. What is more, biologically synthesized ZnO nanoparticles demonstrate high efficiency in treatment of human non-small cell lung cancer A549.
Larvicidal activity and insecticidal mechanism of Chelidonium majus on Lymantria dispar.[Pubmed: 29107235]
Based on the broad spectrum of its biological activities, Chelidonium majus has been studied extensively in the medical field. However, few studies have focused on the insecticidal activity of C. majus, and the precise mechanism of its insecticidal activity. In the present study, larvicidal activity and insecticidal mechanism of C. majus on Lymantria dispar were investigated using bioassays, in vitro and in vivo enzyme activity assays, determination of the nutritional index, and gene transcription analysis. The results showed that alkaloids are the main insecticidal ingredients in C. majus. Among the five isoquinoline alkaloids, coptisine was present at the highest concentration (1624.23mg/L), while tetrahydrocoptisine showed the lowest concentration (0.47mg/L). Both the crude extract of C. majus (CECm) and the total alkaloids of C. majus (TACm) possessed a potent insecticidal activity toward L. dispar larvae. TACm had significant effects on the relative consumption rate, efficiency of conversion of digested food into growth, approximate digestibility, and approximate digestibility of L. dispar larvae. Enzyme activity assays suggested that both CECm and TACm displayed their strongest inhibitory activity to in vitro glutathione S-transferase (GST) and acetylcholinesterase (AChE), and showed the weakest inhibition of in vitro carboxylesterase (CarE). Moreover, CECm and TACm affected the in vivo activities of five enzymes. The in vivo activities of AChE and CarE in L. dispar larvae were inhibited significantly by CECm and TACm. Additionally, qRT-PCR analysis revealed that the transcription of the five enzymes was also affected by TACm. In conclusion, alkaloids in C. majus showed a prominent toxicity to L. dispar by reducing food intake, influencing nutritional indices, and affecting the activity and mRNA transcription of detoxifying and protective enzymes. This study provides novel insights into the insecticidal mechanism of C. majus.
Enrichment of chelidonine from Chelidonium majus L. using macroporous resin and its antifungal activity.[Pubmed: 29102247]
None


