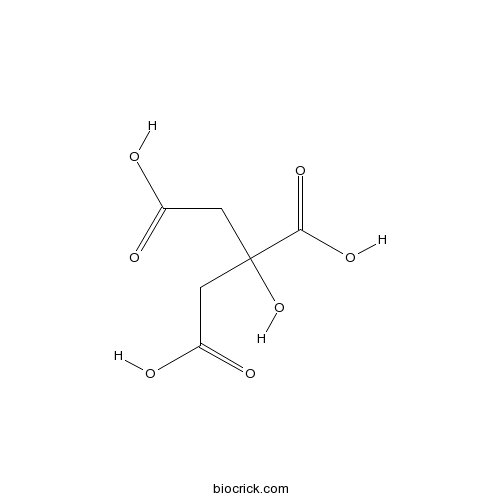
Citric acidCAS# 77-92-9 |
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Batimastat sodium salt
Catalog No.:BCC2075
CAS No.:130464-84-5
- Plumbagin
Catalog No.:BCN2586
CAS No.:481-42-5
- Fluorouracil (Adrucil)
Catalog No.:BCC2135
CAS No.:51-21-8
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- LY2334737
Catalog No.:BCC4060
CAS No.:892128-60-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 77-92-9 | SDF | Download SDF |
| PubChem ID | 311 | Appearance | Powder |
| Formula | C6H8O7 | M.Wt | 192.12 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 150 mg/mL (780.76 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-hydroxypropane-1,2,3-tricarboxylic acid | ||
| SMILES | C(C(=O)O)C(CC(=O)O)(C(=O)O)O | ||
| Standard InChIKey | KRKNYBCHXYNGOX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H8O7/c7-3(8)1-6(13,5(11)12)2-4(9)10/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Citric acid is a natural preservative and food tartness enhancer, it is also an Al-chelating substance and a natural antioxidant. Citric acid (1-2 g/kg) can decrease brain lipid peroxidation and inflammation, liver damage, and DNA fragmentation. Citric acid has phytoremediation of heavy metal contaminated soil; citric acid can decrease the adsorption of both lead and cadmium. Dietary citric acid can effectively improve phytate P utilization in chicks. |
| Targets | TNF-α | Caspase | Antifection |
| In vitro | The role of citric acid on the phytoremediation of heavy metal contaminated soil.[Pubmed: 12688495]Chemosphere. 2003 Feb;50(6):807-11.Adsorption and hydroponics experiments were conducted to study the role of Citric acid on the phytoremediation of heavy metal contaminated soil.
Efficacy of citric acid denture cleanser on the Candida albicans biofilm formed on poly(methyl methacrylate): effects on residual biofilm and recolonization process.[Pubmed: 24957210]BMC Oral Health. 2014 Jun 23;14:77.It is well known that the use of denture cleansers can reduce Candida albicans biofilm accumulation; however, the efficacy of Citric acid denture cleansers is uncertain. In addition, the long-term efficacy of this denture cleanser is not well established, and their effect on residual biofilms is unknown. This in vitro study evaluated the efficacy of Citric acid denture cleanser treatment on C. albicans biofilm recolonization on poly(methyl methacrylate) (PMMA) surface.
Inhibitory impacts of natural antioxidants (ascorbic and citric acid) and vacuum packaging on lipid oxidation in frozen Persian sturgeon fillets[Reference: WebLink]Iranian Journal of Fisheries Sciences, 2010, 9(9):279-292.This study was aimed to investigate effects of aqueous Citric acid (CA) and ascorbic acid (AA)
on lipid oxidation in comparison with effect of vacuum packaging in order to find better
treatment to delay improper changes in the Persian sturgeon (Acipenser persicus) fillets during
frozen storage due to lipid oxidation.
|
| In vivo | Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice.[Pubmed: 24433072]J Med Food. 2014 May;17(5):588-98.Citric acid is a weak organic acid found in the greatest amounts in citrus fruits. This study examined the effect of Citric acid on endotoxin-induced oxidative stress of the brain and liver.
|
| Animal Research | The effects of citric acid on phytate-phosphorus utilization in young chicks and pigs.[Pubmed: 10764076]J Anim Sci. 2000 Mar;78(3):682-9.Several bioassays were conducted with young chicks and pigs fed phosphorus (P)-deficient corn-soybean meal diets.
|

Citric acid Dilution Calculator

Citric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2051 mL | 26.0254 mL | 52.0508 mL | 104.1016 mL | 130.127 mL |
| 5 mM | 1.041 mL | 5.2051 mL | 10.4102 mL | 20.8203 mL | 26.0254 mL |
| 10 mM | 0.5205 mL | 2.6025 mL | 5.2051 mL | 10.4102 mL | 13.0127 mL |
| 50 mM | 0.1041 mL | 0.5205 mL | 1.041 mL | 2.082 mL | 2.6025 mL |
| 100 mM | 0.0521 mL | 0.2603 mL | 0.5205 mL | 1.041 mL | 1.3013 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Citric acid is a weak organic tricarboxylic acid found in citrus fruits. Citric acid is a natural preservative and food tartness enhancer.
In Vitro:Citric acid induces apoptosis through the mitochondrial pathway in the human keratinocyte cell line HaCaT. It inhibits proliferation of HaCaT cells in a dose-dependent manner, but also induces apoptosis and cell cycle-arrest at the G2/M phase (before 24 h) and S phase (after 24 h)[1].
In Vivo:Citric acid is found in all animal tissues as an intermediary substance in oxidative metabolism. The administration of citric acid (1–2 g/kg) attenuates LPS-induced elevations in brain MDA, nitrite, TNF-α, GPx, and PON1 activity. In the liver, nitrite is decreased by 1 g/kg citric acid. Citric acid (1-2 g/kg) decreases brain lipid peroxidation and inflammation, liver damage, and DNA fragmentation[2]. Citric acid supplementation increases intestinal calcium and phosphorus absorption and the retention/intake ratio only in rats fed the 1% Ca diet. Citric acidsupplementation together with a calcium-rich diet allows to obtain an increased retention of calcium and phosphorus in bone. The prolonged administration of calcium citrate supplements may therefore help to increase bone mineral concentration[3]. Oral administration of citric acid ameliorates ketosis and protects against the development of diabetic complications in an animal model of type 1 diabetes[4].
References:
[1]. Ying TH, et al. Citric acid induces cell-cycle arrest and apoptosis of human immortalized keratinocyte cell line (HaCaT) via caspase- and mitochondrial-dependent signaling pathways. Anticancer Res. 2013 Oct;33(10):4411-20.
[2]. Abdel-Salam OM, et al. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J Med Food. 2014 May;17(5):588-98.
[3]. Lacour B, et al. Stimulation by citric acid of calcium and phosphorus bioavailability in rats fed a calcium-rich diet. Miner Electrolyte Metab. 1997;23(2):79-87.
[4]. Nagai R, et al. Citric acid inhibits development of cataracts, proteinuria and ketosis in streptozotocin (type 1) diabetic rats. Biochem Biophys Res Commun. 2010 Feb 26;393(1):118-22.
- Trometamol
Catalog No.:BCC4743
CAS No.:77-86-1
- Tigogenin
Catalog No.:BCN5327
CAS No.:77-60-1
- Tomatidine
Catalog No.:BCN2773
CAS No.:77-59-8
- Cedrol
Catalog No.:BCN8340
CAS No.:77-53-2
- Ursolic acid
Catalog No.:BCN4327
CAS No.:77-52-1
- Chlorthalidone
Catalog No.:BCC4649
CAS No.:77-36-1
- Gibberellins
Catalog No.:BCN2189
CAS No.:77-06-5
- Garcinone C
Catalog No.:BCN4322
CAS No.:76996-27-5
- Euphroside
Catalog No.:BCN6633
CAS No.:76994-07-5
- Notoginsenoside T5
Catalog No.:BCN3727
CAS No.:769932-34-5
- 15-Methoxypinusolidic acid
Catalog No.:BCN4321
CAS No.:769928-72-5
- TPT-260
Catalog No.:BCC5171
CAS No.:769856-81-7
- Triethyl citrate
Catalog No.:BCC9186
CAS No.:77-93-0
- D-(-)-Quinic acid
Catalog No.:BCN1029
CAS No.:77-95-2
- (+,-)-Octopamine HCl
Catalog No.:BCC4814
CAS No.:770-05-8
- Dehydropachymic acid
Catalog No.:BCN3648
CAS No.:77012-31-8
- Hypocrellin A
Catalog No.:BCN3396
CAS No.:77029-83-5
- Knightinol
Catalog No.:BCN1913
CAS No.:77053-06-6
- Acetylknightinol
Catalog No.:BCN1914
CAS No.:77053-07-7
- Gadobutrol
Catalog No.:BCC4164
CAS No.:770691-21-9
- 6-O-trans-Feruloylcatalpol
Catalog No.:BCN4323
CAS No.:125205-48-3
- 2H-1-Benzopyran-5-ol
Catalog No.:BCN3581
CAS No.:770729-34-5
- MK-801 (Dizocilpine)
Catalog No.:BCC4591
CAS No.:77086-21-6
- (+)-MK 801 Maleate
Catalog No.:BCC4014
CAS No.:77086-22-7
The role of citric acid on the phytoremediation of heavy metal contaminated soil.[Pubmed:12688495]
Chemosphere. 2003 Feb;50(6):807-11.
Adsorption and hydroponics experiments were conducted to study the role of Citric acid on the phytoremediation of heavy metal contaminated soil. The results show that addition of Citric acid decreased the adsorption of both lead and cadmium, such an effect was bigger for cadmium than for lead. The decrease in the adsorption of Pb and Cd was mainly due to a decrease of pH in the presence of Citric acid. The presence of Citric acid could alleviate the toxicity of Pb and Cd to radish, and stimulate their transportation from root to shoot. The studies of heavy metal forms using sequential extraction demonstrated that lead was mainly existed as FHAC (a lower bioavailable form) in the root, while F(HCl) was the dominant form in the leaf. The addition of Citric acid to the soil changed the concentration and relative abundance of all the forms. The detoxifying effect of Citric acid to Pb in shoots might result from the transformation of higher toxic forms into lower toxic forms. Cadmium was mainly present as F(NaCl), therefore, it had higher toxicity than lead. The addition of Citric acid increased the abundance of F(H2O) + F(NaCl), indicating that Citric acid treatment could transform cadmium into more transportable forms.
Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice.[Pubmed:24433072]
J Med Food. 2014 May;17(5):588-98.
Citric acid is a weak organic acid found in the greatest amounts in citrus fruits. This study examined the effect of Citric acid on endotoxin-induced oxidative stress of the brain and liver. Mice were challenged with a single intraperitoneal dose of lipopolysaccharide (LPS; 200 mug/kg). Citric acid was given orally at 1, 2, or 4 g/kg at time of endotoxin injection and mice were euthanized 4 h later. LPS induced oxidative stress in the brain and liver tissue, resulting in marked increase in lipid peroxidation (malondialdehyde [MDA]) and nitrite, while significantly decreasing reduced glutathione, glutathione peroxidase (GPx), and paraoxonase 1 (PON1) activity. Tumor necrosis factor-alpha (TNF-alpha) showed a pronounced increase in brain tissue after endotoxin injection. The administration of Citric acid (1-2 g/kg) attenuated LPS-induced elevations in brain MDA, nitrite, TNF-alpha, GPx, and PON1 activity. In the liver, nitrite was decreased by 1 g/kg Citric acid. GPx activity was increased, while PON1 activity was decreased by Citric acid. The LPS-induced liver injury, DNA fragmentation, serum transaminase elevations, caspase-3, and inducible nitric oxide synthase expression were attenuated by 1-2 g/kg Citric acid. DNA fragmentation, however, increased after 4 g/kg Citric acid. Thus in this model of systemic inflammation, Citric acid (1-2 g/kg) decreased brain lipid peroxidation and inflammation, liver damage, and DNA fragmentation.
The effects of citric acid on phytate-phosphorus utilization in young chicks and pigs.[Pubmed:10764076]
J Anim Sci. 2000 Mar;78(3):682-9.
Several bioassays were conducted with young chicks and pigs fed phosphorus (P)-deficient corn-soybean meal diets. With diets for chicks containing .62% Ca and .42% P (.10% available P), graded doses of a Citric acid + sodium citrate (1:1, wt:wt) mixture (0, 1, 2, 4, or 6% of diet) resulted in linear (P < .01) increases in both weight gain and tibia ash. Relative to chicks fed no Citric acid, tibia ash (%) and weight gain (g/d) were increased by 43 and 22%, respectively, in chicks fed 6% Citric acid. Additional chick trials showed that 6% Citric acid alone or sodium citrate alone was as efficacious as the Citric acid + sodium citrate mixture and that 1,450 U/kg of phytase produced a positive response in bone ash and weight gain in chicks fed a diet containing 6% citrate. Varying the Ca:available P ratio with and without citrate supplementation indicated that Citric acid primarily affected phytate-P utilization, not Ca, in chicks. Moreover, chicks did not respond to citrate supplementation when fed a P-deficient (.13% available P), phytate-free casein-dextrose diet. Young pigs averaging 10 to 11 kg also were used to evaluate Citric acid efficacy in two experiments. A P-deficient corn-soybean meal basal diet was used to construct five treatment diets that contained 1) no additive, 2) 3% Citric acid, 3) 6% Citric acid, 4) 1,450 U/kg phytase, and 5) 6% Citric acid + 1,450 U/kg phytase. Phytase supplementation increased (P < .01) weight gain, gain:feed, and metatarsal ash, whereas Citric acid addition increased only gain:feed (P < .05) and metatarsal ash (P < .08). A subsequent 22-d pig experiment was conducted to evaluate the effect of lower levels of Citric acid (0, 1, 2, or 3%) or 1,450 U/kg phytase addition to a P-deficient corn-soybean meal diet. Phytase supplementation improved (P < .01) all criteria measured. Weight gain and gain:feed data suggested a response to Citric acid addition, but this was not supported by fibula ash results (P > .10). The positive responses to phytase were much greater than those to Citric acid in both pig experiments. Thus, dietary Citric acid effectively improved phytate P utilization in chicks but had a much smaller effect in pigs.
Efficacy of citric acid denture cleanser on the Candida albicans biofilm formed on poly(methyl methacrylate): effects on residual biofilm and recolonization process.[Pubmed:24957210]
BMC Oral Health. 2014 Jun 23;14:77.
BACKGROUND: It is well known that the use of denture cleansers can reduce Candida albicans biofilm accumulation; however, the efficacy of Citric acid denture cleansers is uncertain. In addition, the long-term efficacy of this denture cleanser is not well established, and their effect on residual biofilms is unknown. This in vitro study evaluated the efficacy of Citric acid denture cleanser treatment on C. albicans biofilm recolonization on poly(methyl methacrylate) (PMMA) surface. METHODS: C. albicans biofilms were developed for 72 h on PMMA resin specimens (n = 168), which were randomly assigned to 1 of 3 cleansing treatments (CTs) overnight (8 h). CTs included purified water as a control (CTC) and two experimental groups that used either a 1:5 dilution of Citric acid denture cleanser (CT5) or a 1:8 dilution of Citric acid denture cleanser (CT8). Residual biofilms adhering to the specimens were collected and quantified at two time points: immediately after CTs (ICT) and after cleaning and residual biofilm recolonization (RT). Residual biofilms were analyzed by quantifying the viable cells (CFU/mL), and biofilm architecture was evaluated by confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM). Denture cleanser treatments and evaluation periods were considered study factors. Data were analyzed using two-way ANOVA and Tukey's Honestly Significant Difference (HSD) test (alpha = 0.05). RESULTS: Immediately after treatments, Citric acid denture cleansing solutions (CT5 and CT8) reduced the number of viable cells as compared with the control (p < 0.01). However, after 48 h, both CT groups (CT5 and CT8) showed biofilm recolonization (p < 0.01). Residual biofilm recolonization was also detected by CLSM and SEM analysis, which revealed a higher biomass and average biofilm thickness for the CT8 group (p < 0.01). CONCLUSION: Citric acid denture cleansers can reduce C. albicans biofilm accumulation and cell viability. However, this CT did not prevent biofilm recolonization.


