GadobutrolCAS# 770691-21-9 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 770691-21-9 | SDF | Download SDF |
| PubChem ID | 86767262 | Appearance | Powder |
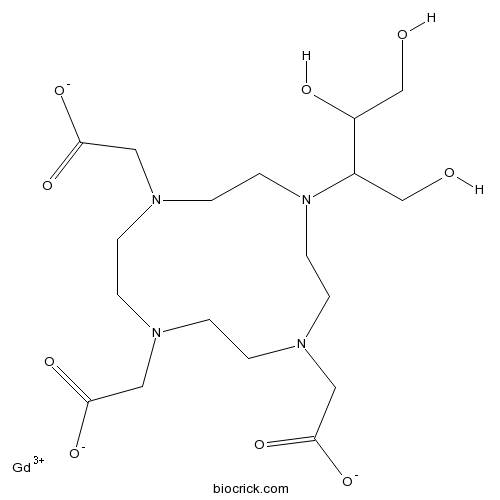
| Formula | C18H31GdN4O9 | M.Wt | 604.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ZK 135079 | ||
| Solubility | H2O : 20 mg/mL (33.07 mM; Need ultrasonic) | ||
| Chemical Name | 2-[4,10-bis(carboxylatomethyl)-7-(1,3,4-trihydroxybutan-2-yl)-1,4,7,10-tetrazacyclododec-1-yl]acetate;gadolinium(3+) | ||
| SMILES | C1CN(CCN(CCN(CCN1CC(=O)[O-])CC(=O)[O-])C(CO)C(CO)O)CC(=O)[O-].[Gd+3] | ||
| Standard InChIKey | ZPDFIIGFYAHNSK-UHFFFAOYSA-K | ||
| Standard InChI | InChI=1S/C18H34N4O9.Gd/c23-12-14(15(25)13-24)22-7-5-20(10-17(28)29)3-1-19(9-16(26)27)2-4-21(6-8-22)11-18(30)31;/h14-15,23-25H,1-13H2,(H,26,27)(H,28,29)(H,30,31);/q;+3/p-3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gadobutrol (Gd-DO3A-butrol; ZK 135079) is a nonionic, paramagnetic contrast agent developed for tissue contrast enhancement in magnetic resonance imaging (MRI).
IC50 value:
Target:
The major chemical differences among these Gd chelates or Gd-based contrast agents (GBCAs) are the presence or absence of overall charge, ionic or nonionic, and their ligand frameworks (linear or macrocyclic). Gd-DO3A-butrol has a macrocyclic framework and is neutral. The DO3A-butrol ligand was developed based on the belief that high overall hydrophilicity of an agent is generally associated with very low protein binding and good biological tolerance. The Gd(III) in Gd-DO3A-butrol has a coordination number of 9. Gd-DO3A-butrol is a water-soluble, highly hydrophilic compound with a partition coefficient between n-butanol and buffer at pH 7.6 of ~ 0.006. Gd-DO3A-butrol is not commercially available in the United States, but it is commercially available in Canada at a concentration of 1.0 mmol/ml (604.72 mg/ml) for contrast enhancement during cranial and spinal imaging with MRI and MR angiography. References: | |||||

Gadobutrol Dilution Calculator

Gadobutrol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6537 mL | 8.2684 mL | 16.5369 mL | 33.0737 mL | 41.3421 mL |
| 5 mM | 0.3307 mL | 1.6537 mL | 3.3074 mL | 6.6147 mL | 8.2684 mL |
| 10 mM | 0.1654 mL | 0.8268 mL | 1.6537 mL | 3.3074 mL | 4.1342 mL |
| 50 mM | 0.0331 mL | 0.1654 mL | 0.3307 mL | 0.6615 mL | 0.8268 mL |
| 100 mM | 0.0165 mL | 0.0827 mL | 0.1654 mL | 0.3307 mL | 0.4134 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gadobutrol (Gd-DO3A-butrol; ZK 135079) is a nonionic, paramagnetic contrast agent developed for tissue contrast enhancement in magnetic resonance imaging (MRI).
- Acetylknightinol
Catalog No.:BCN1914
CAS No.:77053-07-7
- Knightinol
Catalog No.:BCN1913
CAS No.:77053-06-6
- Hypocrellin A
Catalog No.:BCN3396
CAS No.:77029-83-5
- Dehydropachymic acid
Catalog No.:BCN3648
CAS No.:77012-31-8
- (+,-)-Octopamine HCl
Catalog No.:BCC4814
CAS No.:770-05-8
- D-(-)-Quinic acid
Catalog No.:BCN1029
CAS No.:77-95-2
- Triethyl citrate
Catalog No.:BCC9186
CAS No.:77-93-0
- Citric acid
Catalog No.:BCN5374
CAS No.:77-92-9
- Trometamol
Catalog No.:BCC4743
CAS No.:77-86-1
- Tigogenin
Catalog No.:BCN5327
CAS No.:77-60-1
- Tomatidine
Catalog No.:BCN2773
CAS No.:77-59-8
- Cedrol
Catalog No.:BCN8340
CAS No.:77-53-2
- 6-O-trans-Feruloylcatalpol
Catalog No.:BCN4323
CAS No.:125205-48-3
- 2H-1-Benzopyran-5-ol
Catalog No.:BCN3581
CAS No.:770729-34-5
- MK-801 (Dizocilpine)
Catalog No.:BCC4591
CAS No.:77086-21-6
- (+)-MK 801 Maleate
Catalog No.:BCC4014
CAS No.:77086-22-7
- 1H-Indole-3-carboxylic acid
Catalog No.:BCN4324
CAS No.:771-50-6
- Fmoc-Sar-OH
Catalog No.:BCC3338
CAS No.:77128-70-2
- Fmoc-Phe(4-OMe)-OH,Fmoc-Tyr(Me)-OH
Catalog No.:BCC2634
CAS No.:77128-72-4
- Fmoc-N-Me-Phe-OH
Catalog No.:BCC2614
CAS No.:77128-73-5
- SR 57227 hydrochloride
Catalog No.:BCC6967
CAS No.:77145-61-0
- 6alpha-Hydroxycleroda-3,13-dien-16,15-olid-18-oic acid
Catalog No.:BCN1359
CAS No.:771493-42-6
- Spiranine
Catalog No.:BCN2094
CAS No.:77156-23-1
- Spiracine
Catalog No.:BCN2095
CAS No.:77156-24-2
Repeated intravenous administration of gadobutrol does not lead to increased signal intensity on unenhanced T1-weighted images-a voxel-based whole brain analysis.[Pubmed:28289935]
Eur Radiol. 2017 Sep;27(9):3687-3693.
OBJECTIVES: To identify a possible association between repeated intravenous administration of Gadobutrol and increased signal intensity in the grey and white matter using voxel-based whole-brain analysis. METHODS: In this retrospective single-centre study, 217 patients with a clinically isolated syndrome underwent baseline brain magnetic resonance imaging and at least one annual follow-up examination with intravenous administration of 0.1 mmol/kg body weight of Gadobutrol. Using the "Diffeomorphic Anatomical Registration using Exponentiated Lie algebra" (DARTEL) normalisation process, tissue templates for grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) were calculated, as were GM-CSF and WM-CSF ratios. Voxel-based whole-brain analysis was used to calculate the signal intensity for each voxel in each data set. Paired t-test was applied to test differences to baseline MRI for significance. RESULTS: Voxel-based whole-brain analysis demonstrated no significant changes in signal intensity of grey and white matter after up to five Gadobutrol administrations. There was no significant change in GM-CSF and grey WM-CSF ratios. CONCLUSION: Voxel-based whole-brain analysis did not demonstrate increased signal intensity of GM and WM on unenhanced T1-weighted images after repeated Gadobutrol administration. The molecular structure of gadolinium-based contrast agent preparations may be an essential factor causing SI increase on unenhanced T1-weighted images. KEY POINTS: * Repeated administration of Gadobutrol does not lead to increased signal intensity. * Voxel-based whole-brain analysis allows assessment of subtle changes in signal intensity. * Macrocyclic contrast agents in a proven dosage are safe.
Longitudinal Assessment of Dentate Nuclei Relaxometry during Massive Gadobutrol Exposure.[Pubmed:28367903]
Magn Reson Med Sci. 2018 Jan 10;17(1):100-104.
We report the assessment of Dentate Nuclei (DN) R1 (1/T1) and R2* (1/T2*) values in a patient with relapsing-remitting Multiple Sclerosis, exposed to 22 standard (0.1 mmol/kg) doses of Gadobutrol, who underwent eight relaxometric MR measurements within 2 years. DN R1 did not significantly increase nor correlated with cumulative Gadobutrol administration, even after a total dose of 130 ml. Likewise, DN R2* relaxometry remained unchanged. In conclusion, massive Gadobutrol exposure did not induce significant DN relaxometry changes.
Quantitative analysis of late gadolinium enhancement in hypertrophic cardiomyopathy: comparison of diagnostic performance in myocardial fibrosis between gadobutrol and gadopentetate dimeglumine.[Pubmed:28289991]
Int J Cardiovasc Imaging. 2017 Aug;33(8):1191-1200.
The purpose of this study was to compare different semi-automated late gadolinium enhancement (LGE) quantification techniques using Gadobutrol and gadopentetate dimeglumine contrast agents with regard to the diagnosis of fibrotic myocardium in patients with hypertrophic cardiomyopathy (HCM). Thirty patients with HCM underwent two cardiac MRI protocols with use of Gadobutrol and gadopentetate dimeglumine. Contrast-to-noise ratio (CNR) between LGE area and remote myocardium (CNRremote), between LGE area and left ventricular blood pool (CNRpool), and signal-to-noise ratio (SNR) in LGE were compared. The presence and quantity of LGE were determined by visual assessment. With signal threshold versus reference mean (STRM) based thresholds of 2 SD, 5 SD, and 6 SD above the mean signal intensity (SI) of reference myocardium, the full-width at half-maximum (FWHM) technique was used. The volume and segments of the LGE area were compared between the two types of contrast agents. LGE was present in 26 of 30 (86.6%) patients in both protocols. The CNRremote of fibrotic myocardium in Gadobutrol and gadopentetate dimeglumine agents was 26.82 +/- 14.24 and 21.46 +/- 10.59, respectively (P < 0.05). The CNRpool was significantly higher in Gadobutrol (9.32 +/- 7.64 vs. 6.39 +/- 6.11, P < 0.05). The SNR was higher in Gadobutrol (33.36 +/- 14.35 vs. 27.53 +/- 10.91, P < 0.05). The volume of scar size in MR images acquired with Gadobutrol were significantly higher than those with gadopentetate dimeglumine (P < 0.05), and the STRM of 5 SD technique showed the greatest agreement with visual assessment (ICC = 0.99) in both examinations. There was no significant difference in fibrotic segments of the fibrotic myocardium in the LGE area (P < 0.05). This study proved that the Gadobutrol was an effective contrast agent for LGE imaging with superior delineation of fibrotic myocardium as compared to gadopentetate dimeglumine. The 5 SD technique yields the closest approximation of the extent of LGE identified by visual assessment.


