Hedyotis diffusa
Hedyotis diffusa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.

Natural products/compounds from Hedyotis diffusa
- Cat.No. Product Name CAS Number COA
-
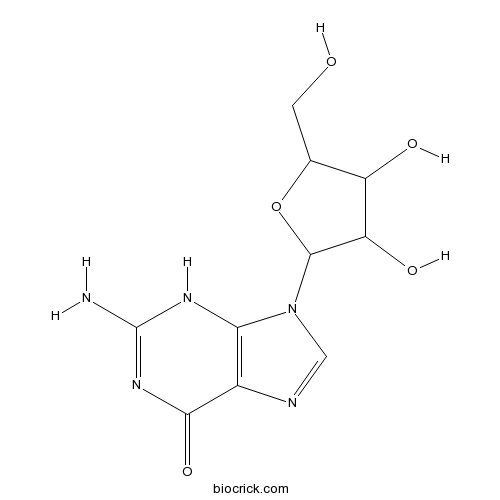
BCN2962
Guanosine118-00-3
Instructions
-
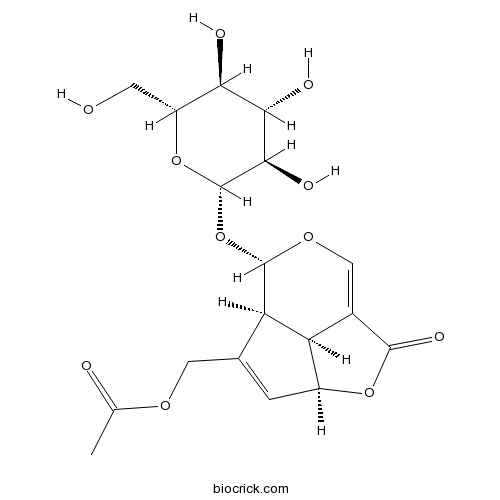
BCN6231
Asperuloside14259-45-1
Instructions
-
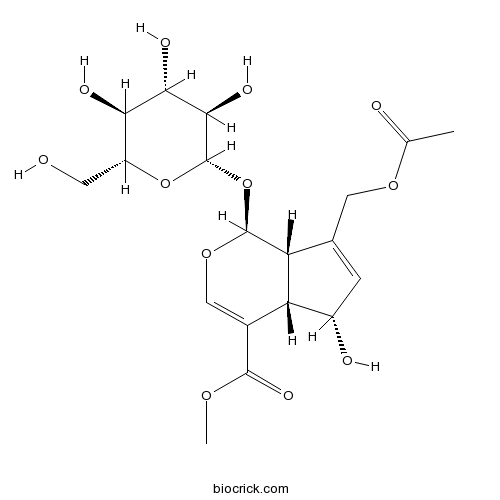
BCN6232
Daphylloside14260-99-2
Instructions
-
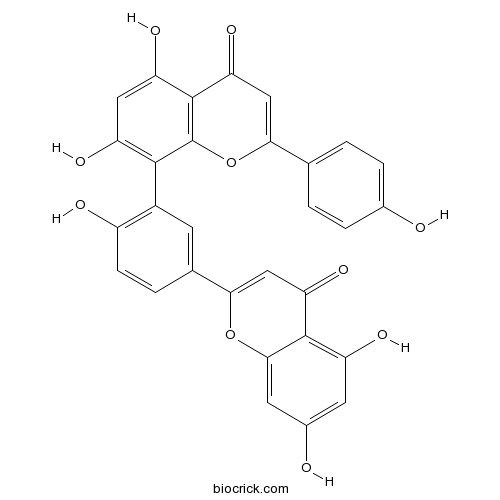
BCN6283
Amentoflavone1617-53-4
Instructions
-
BCN3088
Asperulosidic acid25368-11-0
Instructions
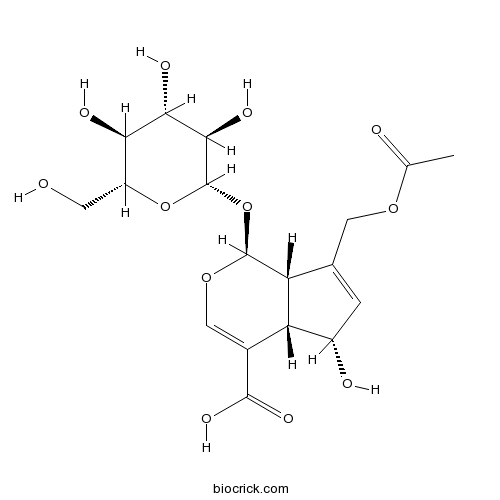
-
BCN2388
Silydianin29782-68-1
Instructions
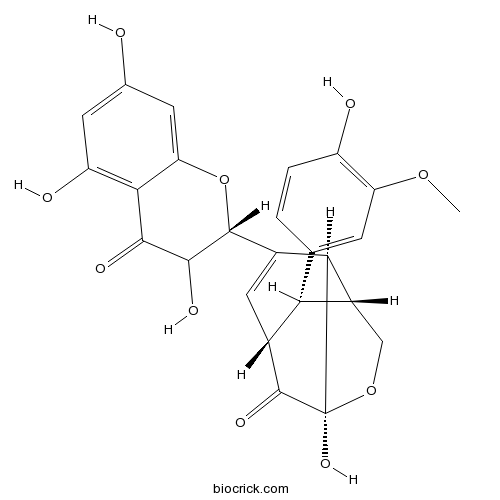
-
BCN5569
Isoquercitrin482-35-9
Instructions
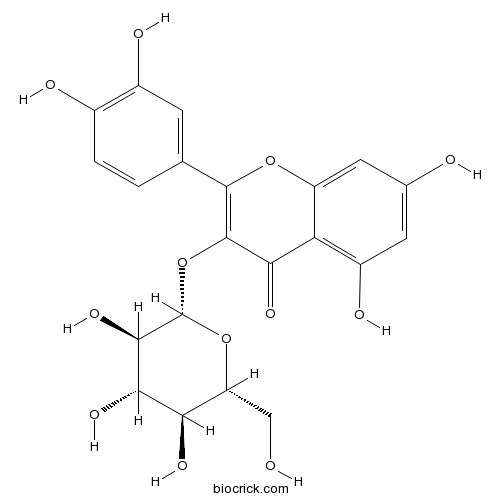
-
BCN5598
Kaempferide491-54-3
Instructions
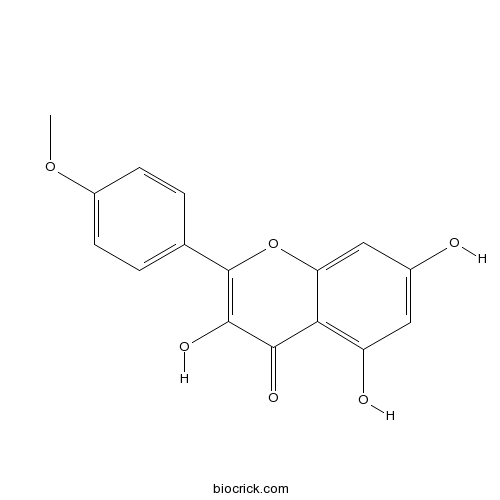
-
BCN5616
Oleanolic acid508-02-1
Instructions

-
BCN5653
Kaempferol520-18-3
Instructions
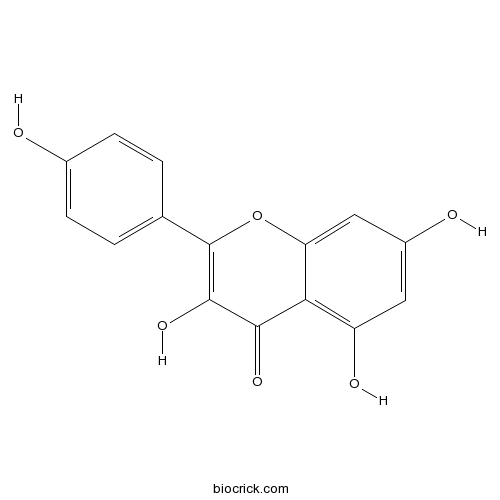
-
BCN1427
Deacetylasperulosidic acid methyl ester52613-28-2
Instructions
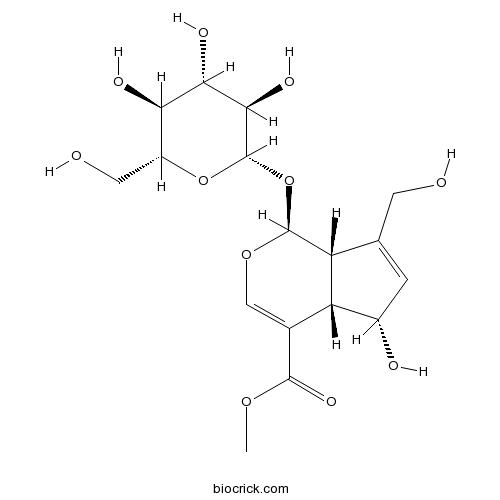
-
BCN3841
Inosine58-63-9
Instructions
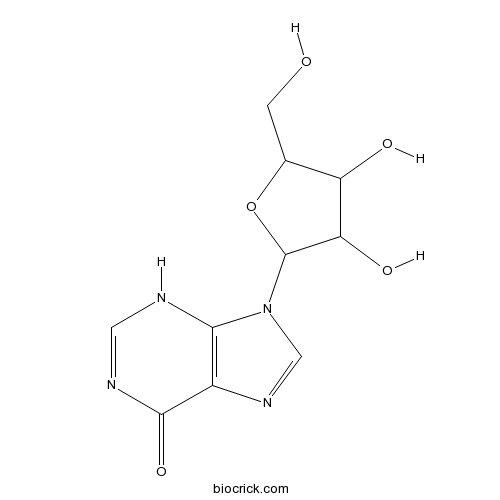
-
BCN3091
2-Hydroxy-1-methoxyanthraquinone6170-06-5
Instructions

-
BCN4327
Ursolic acid77-52-1
Instructions

-
BCN1015
Beta-Sitosterol83-46-5
Instructions
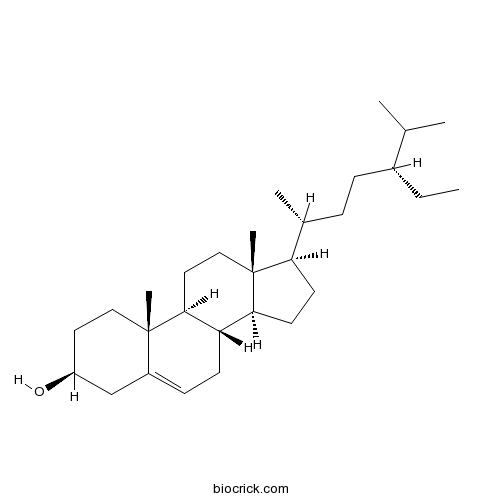
-
BCN4376
Stigmasterol83-48-7
Instructions
-
BCN4546
4-Hydroxybenzoic acid99-96-7
Instructions

Simultaneous determination of fourteen compounds of Hedyotis diffusa Willd extract in rats by UHPLC-MS/MS method: Application to pharmacokinetics and tissue distribution study.[Pubmed: 30048897]
A rapid, sensitive and selective ultra high-performance liquid chromatography-tandem mass spectrometry UHPLC-MS/MS method has been developed and validated for the simultaneous determination of fourteen bioactive ingredients (gallic acid, geniposidic acid, protocatechuic acid, caffeic acid, ferulic acid, scopoletin, apigenin-7-o-glucuronide, daidzein, apigenin, ursolic acid, oleanolic acid, β-sitosterol, coniferin, and stigmasterol) in the plasma and tissues of rats. Danshensu and icariin were used as internal standards (IS1 and IS2). The chromatographic separation was achieved by using an Agilent ZORBAX RRHD Eclipse Plus C18 column (2.1 mm × 50 mm, 1.8 μm) with gradient elution using mobile phase, which consisted of 0.1% acetic acid water (solvent A) and methanol (solvent B) and pumped at a flow rate of 0.3 mL/min. Mass spectrometric detection was performed in multiple reaction monitoring (MRM) mode utilizing electrospray ionization (ESI) in positive and negative mode. The plasma samples were pretreated via protein precipitation with 300 μL of methanol containing 0.1% (v/v) formic acid and organ homogenates were processed by solid-phase extraction (SPE) with Waters Oasis HLB 3 cc (60 mg), respectively. The intra- and inter- day precisions (RSD%) were less than 10.3%, while the accuracy was ranged from -7.34% to 9.10%. Extraction recovery ranged from 85.02 to 112.0% and the matrix effects ranged from 85.12% to 109.6%. The present method exhibited excellent linearity and the lower limits of quantification (LLOQ) were 30.0 ng/mL, 15.0 ng/mL, 80.0 ng/mL, 30.0 ng/mL, 10.0 ng/mL, 3.0 ng/mL, 2.5 ng/mL, 2.5 ng/mL, 1.5 ng/mL, 15.0 ng/mL, 75.0 ng/mL, 15.0 ng/mL, 30.0 ng/mL, and 20.0 ng/mL for gallic acid, protocatechuic acid, geniposidic acid, caffeic acid, ferulic acid, scopoletin, apigenin-7-o-glucuronide, daidzein, apigenin, ursolic acid, oleanolic acid, β-sitosterol, coniferin, and stigmasterol, respectively. This analytical method was verified by the FDA guidelines for bioanalytical method validation and applied to investigate the pharmacokinetics and biodistribution of fourteen constituents of Hedyotis diffusa Willd extract in rats. These results provide useful information for improving the pharmacokinetics and biodistribution of fourteen bioactive ingredients of Hedyotis diffusa Willd extract in SD rats, supporting additional clinical application and Chinese herbal medicine safety evaluations.
Concurrent administration of anticancer chemotherapy drug and herbal medicine on the perspective of pharmacokinetics.[Pubmed: 29703390]
With an increasing number of cancer patients seeking an improved quality of life, complementary and alternative therapies are becoming more common ways to achieve such improvements. The potential risks of concurrent administration are serious and must be addressed. However, comprehensive evidence for the risks and benefits of combining anticancer drugs with traditional herbs is rare. Pharmacokinetic investigations are an efficient way to understand the influence of concomitant remedies. Therefore, this study aimed to collect the results of pharmacokinetic studies relating to the concurrent use of cancer chemotherapy and complementary and alternative therapies. According to the National Health Insurance (NHI) database in Taiwan and several publications, the three most commonly prescribed formulations for cancer patients are Xiang-Sha-Liu-Jun-Zi-Tang, Jia-Wei-Xiao-Yao-San and Bu-Zhong-Yi-Qi-Tang. The three most commonly prescribed single herbs for cancer patients are Hedyotis diffusa, Scutellaria barbata, and Astragalus membranaceus. Few studies have discussed herb-drug interactions involving these herbs from a pharmacokinetics perspective. Here, we reviewed Jia-Wei-Xiao-Yao-San, Long-Dan-Xie-Gan-Tang, Curcuma longa and milk thistle to provide information based on pharmacokinetic evidence for healthcare professionals to use in educating patients about the risks of the concomitant use of various remedies.


