trans-NerolidolCAS# 40716-66-3 |
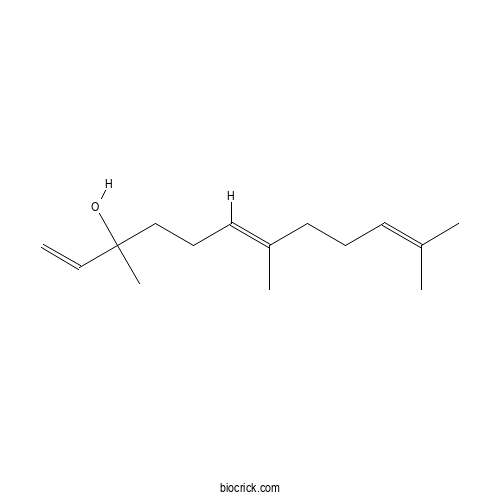
- (+)-Nerolidol
Catalog No.:BCC8219
CAS No.:142-50-7
- Nerolidol
Catalog No.:BCN5459
CAS No.:7212-44-4
- cis-Nerolidol
Catalog No.:BCN9148
CAS No.:3790-78-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40716-66-3 | SDF | Download SDF |
| PubChem ID | 5284507.0 | Appearance | Powder |
| Formula | C15H26O | M.Wt | 222.37 |
| Type of Compound | Aliphatics | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (6E)-3,7,11-trimethyldodeca-1,6,10-trien-3-ol | ||
| SMILES | CC(=CCCC(=CCCC(C)(C=C)O)C)C | ||
| Standard InChIKey | FQTLCLSUCSAZDY-SDNWHVSQSA-N | ||
| Standard InChI | InChI=1S/C15H26O/c1-6-15(5,16)12-8-11-14(4)10-7-9-13(2)3/h6,9,11,16H,1,7-8,10,12H2,2-5H3/b14-11+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

trans-Nerolidol Dilution Calculator

trans-Nerolidol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.497 mL | 22.485 mL | 44.9701 mL | 89.9402 mL | 112.4252 mL |
| 5 mM | 0.8994 mL | 4.497 mL | 8.994 mL | 17.988 mL | 22.485 mL |
| 10 mM | 0.4497 mL | 2.2485 mL | 4.497 mL | 8.994 mL | 11.2425 mL |
| 50 mM | 0.0899 mL | 0.4497 mL | 0.8994 mL | 1.7988 mL | 2.2485 mL |
| 100 mM | 0.045 mL | 0.2249 mL | 0.4497 mL | 0.8994 mL | 1.1243 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hordenine Chloride
Catalog No.:BCX1465
CAS No.:6027-23-2
- Zhebeirine
Catalog No.:BCX1464
CAS No.:143120-47-2
- Demethylwedelolactone sulfate
Catalog No.:BCX1463
CAS No.:1318240-80-0
- 6α-(3-methylvaleryloxy)-Britannilactone
Catalog No.:BCX1462
CAS No.:1260151-66-3
- 6α-isovaleryloxy-Britannilactone
Catalog No.:BCX1461
CAS No.:1259933-04-4
- 6α-isobutyryloxy-Britannilactone
Catalog No.:BCX1460
CAS No.:1259933-02-2
- Puerarin-4'-O-β-D-glucopyranoside
Catalog No.:BCX1459
CAS No.:117047-08-2
- 6α-(2-methybutyryloxy)-Britannilactone
Catalog No.:BCX1458
CAS No.:1260151-65-2
- Teprenone
Catalog No.:BCX1457
CAS No.:6809-52-5
- Fosinopril EP impurity D
Catalog No.:BCX1456
CAS No.:1356353-41-7
- Cirsiumaldehyde
Catalog No.:BCX1455
CAS No.:7389-38-0
- Linustatin
Catalog No.:BCX1454
CAS No.:72229-40-4
- Nor-β-anhydroicaritin
Catalog No.:BCX1467
CAS No.:28610-34-6
- Dehydroandrographolide Succinate Sodium Potasium Salt
Catalog No.:BCX1468
CAS No.:863319-40-8
- 4-Hydroxylonchocarpin
Catalog No.:BCX1469
CAS No.:56083-03-5
- Salicortin
Catalog No.:BCX1470
CAS No.:1887055-63-1
- Iodixanol Impurity E
Catalog No.:BCX1471
CAS No.:255376-57-9
- Ganoderenic acid C2
Catalog No.:BCX1472
CAS No.:1961358-00-8
- Ganoderic acid Gama
Catalog No.:BCX1473
CAS No.:294674-00-3
- Oleuropein Aglycone
Catalog No.:BCX1474
CAS No.:31773-95-2
- (20S)-Panaxatriol
Catalog No.:BCX1475
CAS No.:848830-68-2
- (20S)-Panaxadiol
Catalog No.:BCX1476
CAS No.:112791-34-1
- 20(S)-25-Hydroxyprotopanaxatiol
Catalog No.:BCX1477
CAS No.:113566-83-9
- 20(S)-25-Methoxyprotopanaxadiol
Catalog No.:BCX1478
CAS No.:66007-93-0
Detection of trace components in Xiangdan injection of Dalbergia odorifera based on microextraction and back-extraction along with bar-form-diagram strategy.[Pubmed:38581974]
J Chromatogr A. 2024 May 10;1722:464852.
Xiangdan Injection are commonly used traditional Chinese medicine formulations for the clinical treatment of cardiovascular diseases. However, the trace components of Dalbergia odorifera in Xiangdan Injection pose a challenge for evaluating its quality due to the difficulty of detection. This study proposes a technology combining dispersive liquid-liquid microextraction and back-extraction (DLLME-BE) along with Bar-Form-Diagram (BFD) to address this issue. The proposed combination method involves vortex-mixing tetradecane, which has a lower density than water, with the sample solution to facilitate the transfer of the target components. Subsequently, a new vortex-assisted liquid-liquid extraction step is performed to enrich the components of Dalbergia odorifera in acetonitrile. The sample analysis was performed on HPLC-DAD, and a clear overview of the chemical composition was obtained by integrating spectral and chromatographic information using BFD. The combination of BFD and CRITIC-TOPSIS strategies was used to optimize the process parameters of DLLME-BE. The determined optimal sample pre-treatment process parameters were as follows: 200 muL extraction solvent, 60 s extraction time, 50 muL back-extraction solvent, and 90 s back-extraction time. Based on the above strategy, a total of 29 trace components, including trans-Nerolidol, were detected in the Xiangdan Injection. This combination technology provides valuable guidance for the enrichment analysis of trace components in traditional Chinese medicines.
A novel FCTF evaluation and prediction model for food efficacy based on association rule mining.[Pubmed:37701374]
Front Nutr. 2023 Aug 28;10:1170084.
INTRODUCTION: Food-components-target-function (FCTF) is an evaluation and prediction model based on association rule mining (ARM) and network interaction analysis, which is an innovative exploration of interdisciplinary integration in the food field. METHODS: Using the components as the basis, the targets and functions are comprehensively explored in various databases and platforms under the guidance of the ARM concept. The focused active components, key targets and preferred efficacy are then analyzed by different interaction calculations. The FCTF model is particularly suitable for preliminary studies of medicinal plants in remote and poor areas. RESULTS: The FCTF model of the local medicinal food Laoxianghuang focuses on the efficacy of digestive system cancers and neurological diseases, with key targets ACE, PTGS2, CYP2C19 and corresponding active components citronellal, trans-Nerolidol, linalool, geraniol, alpha-terpineol, cadinene and alpha-pinene. DISCUSSION: Centuries of traditional experience point to the efficacy of Laoxianghuang in alleviating digestive disorders, and our established FCTF model of Laoxianghuang not only demonstrates this but also extends to its possible adjunctive efficacy in neurological diseases, which deserves later exploration. The FCTF model is based on the main line of components to target and efficacy and optimizes the research level from different dimensions and aspects of interaction analysis, hoping to make some contribution to the future development of the food discipline.
Bioactivity of select essential oil constituents against life stages of Anopheles arabiensis (Diptera: Culicidae).[Pubmed:37330107]
Exp Parasitol. 2023 Aug;251:108569.
Malaria is transmitted by infected female Anopheles mosquitoes, and An. arabiensis is a main malaria vector in arid African countries. Like other anophelines, its life cycle comprises of three aquatic stages; egg, larva, and pupa, followed by a free flying adult stage. Current vector control interventions using synthetic insecticides target these stages using adulticides or less commonly, larvicides. With escalating insecticide resistance against almost all conventional insecticides, identification of agents that simultaneously act at multiple stages of Anopheles life cycle presents a cost-effective opportunity. A further cost-effective approach would be the discovery of such insecticides from natural origin. Interestingly, essential oils present as potential sources of cost-effective and eco-friendly bioinsecticides. This study aimed to identify essential oil constituents (EOCs) with potential toxic effects against multiple stages of An. arabiensis life cycle. Five EOCs were assessed for inhibition of Anopheles egg hatching and ability to kill larvae, pupae and adult mosquitoes of An. arabiensis species. One of these EOCs, namely methyleugenol, exhibited potent Anopheles egg hatchability inhibition with an IC(50) value of 0.51 +/- 0.03 muM compared to propoxur (IC(50): 5.13 +/- 0.62 muM). Structure-activity relationship study revealed that methyleugenol and propoxur share a 1,2-dimethoxybenze moiety that may be responsible for the observed egg-hatchability inhibition. On the other hand, all five EOCs exhibited potent larvicidal activity with LC(50) values less than 5 muM, with four of them; cis-nerolidol, trans-Nerolidol, (-)-alpha-bisabolol, and farnesol, also possessing potent pupicidal effects (LC(50) < 5 muM). Finally, all EOCs showed only moderate lethality against adult mosquitoes. This study reports for the first time, methyleugenol, (-)-alpha-bisabolol and farnesol as potent bioinsecticides against early life stages of An. arabiensis. This synchronized activity against Anopheles aquatic stages shows a prospect to integrate EOCs into existing adulticide-based vector control interventions.
High-Yield Biosynthesis of trans-Nerolidol from Sugar and Glycerol.[Pubmed:37148252]
J Agric Food Chem. 2023 Jun 7;71(22):8479-8487.
Isoprenoids, or terpenoids, have wide applications in food, feed, pharmaceutical, and cosmetic industries. Nerolidol, an acyclic C15 isoprenoid, is widely used in cosmetics, food, and personal care products. Current supply of nerolidol is mainly from plant extraction that is inefficient, costly, and of inconsistent quality. Here, we screened various nerolidol synthases from bacteria, fungi, and plants and found that the strawberry nerolidol synthase was most active in Escherichia coli. Through systematic optimization of the biosynthetic pathways, carbon sources, inducer, and genome editing, we constructed a series of deletion strains (single mutants DeltaldhA, DeltapoxB, DeltapflB, and DeltatnaA; double mutants DeltaadhE-DeltaldhA; and triple mutants and beyond DeltaadhE-DeltaldhA-DeltapflB and DeltaadhE-DeltaldhA-DeltaackA-pta) that produced high yields of 100% trans-Nerolidol. In flasks, the highest nerolidol titers were 1.8 and 3.3 g/L in glucose-only and glucose-lactose-glycerol media, respectively. The highest yield reached 26.2% (g/g), >90% of the theoretic yield. In two-phase extractive fed-batch fermentation, our strain produced approximately 16 g/L nerolidol within 4 days with about 9% carbon yield (g/g). In a single-phase fed-batch fermentation, the strain produced >6.8 g/L nerolidol in 3 days. To the best of our knowledge, our titers and productivity are the highest in the literature, paving the way for future commercialization and inspiring biosynthesis of other isoprenoids.
Effects of processing procedures on the formation of aroma intensity and odor characteristic of Benshan tea (Oolong tea, Camellia sentences).[Pubmed:37025800]
Heliyon. 2023 Mar 25;9(4):e14855.
Benshan tea is a kind of oolong tea, and Benshan (Camellia sinensis) tea tree originates from Anxi County of Fujian Province in China, which is a national tea tree breed. Tea processing is the key to the formation of its odor characteristics. It is extremely important to step by step analyze effects of tea processing on aroma intensity and the formation of odor characteristics for optimizing tea processing process and improving tea quality. The results of this study showed that processing resulted in a significant increase in the content of volatile compounds in tea leaves, i.e., from 25.213 mug/kg to 111.223 mug/kg, in which the volatile compounds were mainly terpenoids. Secondly, the analysis found that 20 kinds of key compounds constituted to odor characteristics of Benshan tea leaves, among which geraniol, trans-beta-ionone, gerol, citronellol, benzeneacetaldehyde, and trans-Nerolidol were the most key six. Floral and fruity aromas, especially floral aroma, mainly formed odor characteristics of Benshan tea after processing, while floral aroma mainly came from the contribution of geraniol, which was the foremost compound in the formation of floral aroma of Benshan tea.
A Brief Review on Murraya paniculata (Orange Jasmine): pharmacognosy, phytochemistry and ethanomedicinal uses.[Pubmed:37007290]
J Pharmacopuncture. 2023 Mar 31;26(1):10-17.
OBJECTIVES: Murraya paniculata (family-Rutaceae), popularly known as orange jasmine, is the most important evergreen plant. The Rutaceae family is economically significant due to its diverse edible fruits and essential oils. METHODS: Murraya paniculata extracts (MPE) of leaf have been shown to include phenolic compounds, highly oxygenated flavonoids, flavanones, sesquiterpenoids, polymethoxy glycosides, and coumarins. Cyclocitral, methyl salicylate, trans-Nerolidol, cubenol, isogermacrene, -cadinol, and cubeb-11-ene are all abundant in MPE. The usages of various parts of this plant, such as bark, leaves and flower, as a remedy for a variety of ailments as widely recorded in the traditional literature. The plant has anti-diabetic, anti-obesity, antibacterial, anti-implantation, anti-oxidative, cytotoxic, anti-diarrheal, antidepressant and anti-anxiety properties and many others. RESULTS: The goal of the review is to reignite interest in this potential plant, encouraging researchers to continue their research in order to uncover novel therapeutic compounds for the treatment and management of a range of infections. The current review provided a comprehensive overview of this traditional unique plant. CONCLUSION: The review paves a way for exploring its active chemical elements with substantial pharmacological values further for potential benefits of mankind.
The insecticidal activity of essential oil constituents against pyrethroid-resistant Anopheles funestus (Diptera: Culicidae).[Pubmed:36898498]
Parasitol Int. 2023 Aug;95:102749.
Malaria vector control relies on the use of insecticides for indoor residual spraying and long-lasting bed nets. However, insecticide resistance to pyrethroids among others, has escalated. Anopheles funestus, one of the major African malaria vectors, has attained significant levels of resistance to pyrethroids. Overexpressed P450 monooxygenases have been previously identified in pyrethroid resistant An. funestus. The escalating resistance against conventional insecticides signals an urgent need for identification of novel insecticides. Essential oils have gained recognition as promising sources of alternative natural insecticides. This study investigated six essential oil constituents, farnesol, (-)-alpha-bisabolol, cis-nerolidol, trans-Nerolidol, methyleugenol, santalol (alpha and beta isomers) and essential oil of sandalwood, for the adulticidal effects against pyrethroid-resistant An. funestus strain. The susceptibility against these terpenoids were evaluated on both pyrethroid-susceptible and resistant An. funestus. Furthermore, the presence of overexpressed monooxygenases in resistant An. funestus was confirmed. Results showed that both the pyrethroid-susceptible and resistant An. funestus were susceptible to three EOCs; cis-nerolidol, trans-Nerolidol and methyleugenol. On the other hand, the pyrethroid-resistant An. funestus survived exposure to both farnesol and (-)-alpha-bisabolol. This study however does not show any direct association of the overexpressed Anopheles monooxygenases and the efficacy of farnesol and (-)-alpha-bisabolol. The enhanced activity of these terpenoids against resistant An. funestus that has been pre-exposed to a synergist, piperonyl butoxide, suggests their potential effectiveness in combination with monooxygenase inhibitors. This study proposes that cis-nerolidol, trans-Nerolidol and methyleugenol are potential agents for further investigation as novel bioinsecticides against pyrethroid-resistant An. funestus strain.
Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora.[Pubmed:36770639]
Molecules. 2023 Jan 18;28(3):973.
Cinnamomum camphora is a traditional aromatic plant used to produce linalool and borneol flavors in southern China; however, its leaves also contain many other unutilized essential oils. Herein, we report geographic relationships for the yield and compositional diversity of C. camphora essential oils. The essential oils of 974 individual trees from 35 populations in 13 provinces were extracted by hydrodistillation and analyzed qualitatively and quantitatively by gas chromatography-mass spectrometry and gas chromatography-flame ionization detection, respectively. Oil yields ranged from 0.01% to 3.46%, with a significantly positive correlation with latitude and a significantly negative correlation with longitude. In total, 41 compounds were identified, including 15 monoterpenoids, 24 sesquiterpenoids, and two phenylpropanoids. Essential oil compositions varied significantly among individuals and could be categorized into various chemotypes. The six main chemotypes were eucalyptol, nerolidol, camphor, linalool, selina, and mixed types. The other 17 individual plants were chemotypically rare and exhibited high levels of methyl isoeugenol, methyl eugenol, delta-selinene, or borneol. Eucalyptol-type plants had the highest average oil yield of 1.64%, followed in decreasing order by linalool-, camphor-, mixed-, selina-, and nerolidol-type plants. In addition, the five main compounds exhibited a clear geographic gradient. Eucalyptol and linalool showed a significantly positive correlation with latitude, while selina-6-en-4-ol was significantly and negatively correlated with latitude. trans-Nerolidol and selina-6-en-4-ol showed significantly positive correlations with longitude, whereas camphor was significantly and negatively correlated with longitude. Canonical correspondence analysis indicated that environmental factors could strong effect the oil yield and essential oil profile of C. camphora.
In vitro and in silico analysis of the Anopheles anticholinesterase activity of terpenoids.[Pubmed:36455706]
Parasitol Int. 2023 Apr;93:102713.
Anopheles gambiae, An. coluzzii, An. arabiensis, and An. funestus are major vectors in high malaria endemic African regions. Various terpenoid classes form the main chemical constituent repository of essential oils, many of which have been shown to possess insecticidal effects against Anopheles species. The current study aimed to assess the bioactivity of terpenoids including four sesquiterpene alcohols, farnesol, (-)-alpha-bisabolol, cis-nerolidol, and trans-Nerolidol; a phenylpropanoid, methyleugenol, and a monoterpene, (R)-(+)-limonene, using the larvicidal screening assay against the four Anopheles species. The mechanism of action was investigated through in vitro acetylcholinesterase inhibition assay and in silico molecular modelling. All six terpenoids showed potent larvicidal activity against the four Anopheles species. Insights into the mechanism of action revealed that the six terpenoids are strong AChE inhibitors against An. funestus and An. arabiensis, while there was a moderate inhibitory activity against An. gambiae AChE, but very weak activity against An. coluzzii. Interestingly, in the in silico study, farnesol established a favourable hydrogen bonding interaction with a conserved amino acid residue, Cys(447), at the entrance to the active site gorge. While (-)-alpha-bisabolol and methyleugenol displayed a strong interaction with the catalytic Ser(360) and adjacent amino acid residues; but sparing the mutable Gly(280) residue that confers resistance to the current anticholinesterase insecticides. As a result, this study identified farnesol, (-)-alpha-bisabolol, and methyleugenol as selective bioinsecticidal agents with potent Anopheles AChE inhibition. These terpenoids present as natural compounds for further development as anticholinesterase bioinsecticides.
Enhancing Trans-Nerolidol Productivity in Yarrowia lipolytica by Improving Precursor Supply and Optimizing Nerolidol Synthase Activity.[Pubmed:36444843]
J Agric Food Chem. 2022 Dec 7;70(48):15157-15165.
The low enzymatic capability of terpene synthases and the limited availability of precursors often hinder the productivity of terpenes in microbial hosts. Herein, a systematic approach combining protein engineering and pathway compartmentation was exploited in Yarrowia lipolytica for the high-efficient production of trans-Nerolidol, a sesquiterpene with various commercial applications. Through the single-gene overexpression, the reaction catalyzed by nerolidol synthase (FaNES1) was identified as another rate-limiting step. An optimized FaNES1(G498Q) was then designed by rational protein engineering using homology modeling and docking studies. Additionally, further improvement of trans-Nerolidol production was observed as enhancing the expression of an endogenous carnitine acetyltransferase (CAT2) putatively responsible for acetyl-CoA shuttling between peroxisome and cytosol. To harness the peroxisomal acetyl-CoA pool, a parallel peroxisomal pathway starting with acetyl-CoA to trans-Nerolidol was engineered. Finally, the highest reported titer of 11.1 g/L trans-Nerolidol in the Y. lipolytica platform was achieved in 5 L fed-batch fermentation with the carbon restriction approach.
Evaluation of the anti-inflammatory effects of selected cannabinoids and terpenes from Cannabis Sativa employing human primary leukocytes.[Pubmed:36228902]
Food Chem Toxicol. 2022 Dec;170:113458.
Cannabis is well established as possessing immune modulating activity. The objective of this study was to evaluate the anti-inflammatory properties of selected cannabis-derived terpenes and cannabinoids. Based on their activity in cannabis-chemovar studies, alpha-pinene, trans-Nerolidol, D-limonene, linalool and phytol were the selected terpenes evaluated. The cannabinoid compounds evaluated included cannabidivarin, cannabidiol, cannabinol, cannabichromene, cannabigerol and delta-9-tetrahydrocannabinol. Human PBMC were pretreated with each compound, individually, at concentrations extending from 0.001 to 10 muM and then stimulated with CpG (plasmacytoid dendritic cell), LPS (monocytes), or anti-CD3/CD28 (T cells). Proliferation, activation marker expression, cytokine production and phagocytosis, were quantified. Of the 21 responses assayed for each compound, cannabinoids showed the greatest immune modulating activity compared to their vehicle control. Delta-9-tetrahydrocannabinol possessed the greatest activity affecting 11 immune parameters followed by cannabidivarin, cannabigerol, cannabichromene, cannabinol and cannabidiol. alpha-Pinene showed the greatest immune modulating activity from the selected group of terpenes, followed by linalool, phytol, trans-Nerolidol. Limonene had no effect on any of the parameters tested. Overall, these studies suggest that selected cannabis-derived terpenes displayed minimal immunological activity, while cannabinoids exhibited a broader range of activity. Compounds possessing anti-inflammatory effects may be useful in decreasing inflammation associated with a range of disorders, including neurodegenerative disorders.
Dynamic Changes of Volatile Compounds during the Xinyang Maojian Green Tea Manufacturing at an Industrial Scale.[Pubmed:36076866]
Foods. 2022 Sep 2;11(17):2682.
Xinyang Maojian (XYMJ) is one of the premium green teas and originates from Xinyang, which is the northernmost green tea production area in China. The special geographic location, environmental conditions, and manufacturing process contribute to the unique flavor and rich nutrition of XYMJ green tea. Aroma is an important quality indicator in XYMJ green tea. In order to illustrate the aroma of XYMJ green tea, the key odorants in XYMJ green tea and their dynamic changes during the manufacturing processes were analyzed by headspace solid-phase microextraction (HS-SPME) combined with gas chromatography-mass spectrometry (GC-MS). A total of 73 volatile compounds of six different chemical classes were identified in the processed XYMJ green tea samples, and the manufacturing processes resulted in the losses of total volatile compounds. Among the identified volatile compounds, twenty-four aroma-active compounds, such as trans-Nerolidol, geranylacetone, nonanal, (+)-delta-cadinene, linalool, (Z)-jasmone, cis-3-hexenyl butyrate, cis-3-hexenyl hexanoate, methyl jasmonate, and beta-ocimene, were identified as the key odorants of XYMJ green tea based on odor activity value (OAV). The key odorants are mainly volatile terpenes (VTs) and fatty acid-derived volatiles (FADVs). Except for (+)-delta-cadinene, copaene, cis-beta-farnesene, (Z,E)-alpha-farnesene and phytol acetate, the key odorants significantly decreased after fixing. The principal coordinate analysis (PCoA) and the hierarchical cluster analysis (HCA) analyses suggested that fixing was the most important manufacturing process for the aroma formation of XYMJ green tea. These findings of this study provide meaningful information for the manufacturing and quality control of XYMJ green tea.
Plant Volatile Compounds of the Invasive Alligatorweed, Alternanthera philoxeroides (Mart.) Griseb, Infested by Agasicles hygrophila Selman and Vogt (Coleoptera: Chrysomelidae).[Pubmed:36013435]
Life (Basel). 2022 Aug 17;12(8):1257.
Plants release a variety of volatiles and herbivore-induced plant volatiles (HIPVs) after being damaged by herbivorous insects, which play multiple roles in the interactions with other plants and insects. Agasicles hygrophila Selman and Vogt (Coleoptera: Chrysomelidae) is a monophagous natural enemy and an effective biocontrol agent for Alternanthera philoxeroides (Mart.) Griseb. Here, we reported differences among the volatiles of A. philoxeroides by solid phase microextraction (SPME) using a gas chromatography-mass spectrometer (GC-MS). We compared the volatile emission of: (1) clean plants (CK); (2) A. philoxeroides plants with mechanical damage treatment (MD); and (3) A. philoxeroides plants infested with A. hygrophila 1st, 2nd, and 3rd larvae and female and male adults. A total of 97 volatiles were recorded, of which 5 occurred consistently in all treatments, while 61 volatiles were only observed in A. philoxeroides infested by A. hygrophila, such as trans-Nerolidol, (E)-beta-farnesene, and (3E,7E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (E, E-TMTT), etc. Among the 97 volatile compounds, 37 compounds belong to alkenes, 29 compounds belong to alkanes, and there were 8 esters, 8 alcohols and 6 ketones. Orthogonal partial least squares-discrimination analysis (OPLS-DA) showed that the different treatments were separated from each other, especially insect feeding from CK and MD treatments, and 19 volatiles contributed most to the separation among the treatments, with variable importance for the projection (VIP) values > 1. Our findings indicated that the alligatorweed plants could be induced to release volatiles by different stages of A. hygrophila, and the volatile compounds released differ quantitatively and qualitatively. The results from this study laid an important foundation for using volatile organic compounds (VOCs) and HIPVs of alligatorweed to improve the control effect of A. hygrophila on A. philoxeroides.
Indigenous Non-Saccharomyces Yeasts With beta-Glucosidase Activity in Sequential Fermentation With Saccharomyces cerevisiae: A Strategy to Improve the Volatile Composition and Sensory Characteristics of Wines.[Pubmed:35633724]
Front Microbiol. 2022 May 12;13:845837.
Non-Saccharomyces (NS) yeasts with high beta-glucosidase activity play a vital role in improving the aroma complexity of wines by releasing aroma compounds from glycosidic precursors during fermentation. In this study, the effect of sequential inoculation fermentation of Meyerozyma guilliermondii NM218 and Hanseniaspora uvarum BF345 with two Saccharomyces cerevisiae strains [Vintage Red (VR) and Aroma White (AW)] on volatile compounds and sensory characteristics of wines was investigated. Prior to winemaking trials, the sequential inoculation times of the two NS yeasts were evaluated in synthetic must, based on changes in strain population and enzyme activity. The intervals for inoculation of NM218 and BF345 with the S. cerevisiae strains were 48 and 24 h, respectively. In the main experiment, sequential inoculation fermentations of the two strains with S. cerevisiae were carried out in Cabernet Sauvignon (CS) and Chardonnay (CH) grape must. The oenological parameters, volatile composition, and sensory characteristics of the final wines were assessed. No clear differences were observed in the oenological parameters of the sequentially fermented CH wines compared with the control, except for residual sugar and alcohol. However, in CS wines, the total acid contents were significantly lower in the wines fermented by sequential inoculation compared to the control. Both NM218 and BF345 improved the aroma complexity of wines by increasing esters and terpenes when inoculated with S. cerevisiae strains compared to inoculation with S. cerevisiae strains alone. NM218 resulted in a more positive effect on CS wine aroma, with higher levels of citronellol and trans-Nerolidol. BF345 significantly enhanced the floral and fruity aromas of CH wine by producing higher concentrations of geranyl acetone, beta-damascenone, trans-Nerolidol, and nerol. Both NM218 and BF345 yeasts could potentially be used to improve wine aroma and overall quality, especially wine floral and fruity aromas, when used in sequential inoculation with S. cerevisiae.


