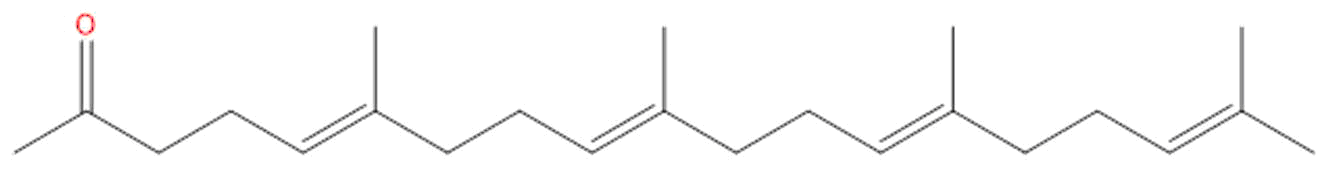
TeprenoneCAS# 6809-52-5 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 6809-52-5 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C23H38O | M.Wt | 330.56 |
| Type of Compound | Aliphatics | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Teprenone Dilution Calculator

Teprenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0252 mL | 15.1258 mL | 30.2517 mL | 60.5034 mL | 75.6292 mL |
| 5 mM | 0.605 mL | 3.0252 mL | 6.0503 mL | 12.1007 mL | 15.1258 mL |
| 10 mM | 0.3025 mL | 1.5126 mL | 3.0252 mL | 6.0503 mL | 7.5629 mL |
| 50 mM | 0.0605 mL | 0.3025 mL | 0.605 mL | 1.2101 mL | 1.5126 mL |
| 100 mM | 0.0303 mL | 0.1513 mL | 0.3025 mL | 0.605 mL | 0.7563 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fosinopril EP impurity D
Catalog No.:BCX1456
CAS No.:1356353-41-7
- Cirsiumaldehyde
Catalog No.:BCX1455
CAS No.:7389-38-0
- Linustatin
Catalog No.:BCX1454
CAS No.:72229-40-4
- Liraglutide
Catalog No.:BCX1453
CAS No.:204656-20-2
- 2'-Acetyltaxol
Catalog No.:BCX1452
CAS No.:92950-40-8
- Methyl cis-11-eicosenoate
Catalog No.:BCX1451
CAS No.:2390-09-2
- Stigmastanol
Catalog No.:BCX1450
CAS No.:83-45-4
- 16,17-Didehydroganoderic acid D
Catalog No.:BCX1449
CAS No.:1427189-02-3
- 4-Deoxyphorbol
Catalog No.:BCX1448
CAS No.:79083-67-3
- Neotame
Catalog No.:BCX1447
CAS No.:165450-17-9
- 6-Carboxyl-7-hydroxy-2,3-dimethylchromone
Catalog No.:BCX1446
CAS No.:108170-57-6
- 1β-Methoxydiversifolin
Catalog No.:BCX1445
CAS No.:110382-36-0
- 6α-(2-methybutyryloxy)-Britannilactone
Catalog No.:BCX1458
CAS No.:1260151-65-2
- Puerarin-4'-O-β-D-glucopyranoside
Catalog No.:BCX1459
CAS No.:117047-08-2
- 6α-isobutyryloxy-Britannilactone
Catalog No.:BCX1460
CAS No.:1259933-02-2
- 6α-isovaleryloxy-Britannilactone
Catalog No.:BCX1461
CAS No.:1259933-04-4
- 6α-(3-methylvaleryloxy)-Britannilactone
Catalog No.:BCX1462
CAS No.:1260151-66-3
- Demethylwedelolactone sulfate
Catalog No.:BCX1463
CAS No.:1318240-80-0
- Zhebeirine
Catalog No.:BCX1464
CAS No.:143120-47-2
- Hordenine Chloride
Catalog No.:BCX1465
CAS No.:6027-23-2
- trans-Nerolidol
Catalog No.:BCX1466
CAS No.:40716-66-3
- Nor-β-anhydroicaritin
Catalog No.:BCX1467
CAS No.:28610-34-6
- Dehydroandrographolide Succinate Sodium Potasium Salt
Catalog No.:BCX1468
CAS No.:863319-40-8
- 4-Hydroxylonchocarpin
Catalog No.:BCX1469
CAS No.:56083-03-5
The protective effect of teprenone in TNBS-induced ulcerative colitis rats by modulating the gut microbiota and reducing inflammatory response.[Pubmed:38252282]
Immunopharmacol Immunotoxicol. 2024 Apr;46(2):255-263.
OBJECTIVE: Ulcerative colitis (UC), a chronic and refractory nonspecific inflammatory bowel disease, affects millions of patients worldwide and increases the risk of colorectal cancer. Teprenone is an acylic polyisoprenoid that exerts anti-inflammatory properties in rat models of peptic ulcer disease. This in vitro and in vivo study was designed to investigate the effects of Teprenone on UC and to explore the underlying mechanisms. METHODS: Human intestinal epithelial cells (Caco-2 cells) serve as the in vitro experimental model. Lipopolysaccharide (LPS, 1 mug/mL) was employed to stimulate the production of pro-inflammatory cytokines (interleukin [IL]-6, IL-1beta, and tumor necrosis factor [TNF]-alpha), Toll-like receptor-4 (TLR4), MyD88 expression, and NF-kappaB activation. A trinitrobenzene sulfonic acid (TNBS)-induced chronic UC rat model was employed for the in vivo assay. RESULTS: Pro-inflammatory cytokine stimulation by LPS in Caco-2 cells was inhibited by Teprenone at 40 mug/mL through the TLR4/NF-kappaB signaling pathway. Teprenone attenuated TNBS-induced UC, decreased myeloperoxidase and malondialdehyde, induced TLR4 expression and NF-kappaB activation, and increased glutathione and zonula occludens-1 level in the rat colonic tissue. Moreover, Fusobacterium, Escherichia coli, Porphyromonas gingivalis elevation, and Mogibacterium timidum decline in UC rats were inhibited by Teprenone. CONCLUSION: Based on our results, the protective effects of Teprenone for UC may be related to its ability to modulate the gut microbiota and reduce the inflammatory response.
Pantoprazole and Vonoprazan Performed Well in Preventing Peptic Ulcer Recurrence in Low-Dose Aspirin Users.[Pubmed:38252210]
Dig Dis Sci. 2024 Mar;69(3):670-682.
BACKGROUND: Low-dose aspirin (LDA) administration is associated with an elevated risk of recurring peptic ulcer (PU) and gastrointestinal (GI) hemorrhage. AIMS: This systematic review and Bayesian network meta-analysis aimed to comprehensively assess the effectiveness of diverse medications in preventing the recurrence of PU and GI hemorrhage in patients with a history of PU receiving long-term LDA therapy. METHODS: This systematic review and network meta-analysis followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and was registered on PROSPERO (CRD42023406550). We searched relevant studies in main databases from inception to March 2023. All statistical analyses were performed using R (version 4.1.3), with the "Gemtc" (version 1.0-1) package. The pooled risk ratio (RR), corresponding 95% credible interval (95% CrI), and the surface under the cumulative ranking curve (SUCRA) were calculated. RESULTS: 11 Randomized clinical trials (RCTs) were included. The analysis underscored pantoprazole was the most efficacious for reducing the risk of PU recurrence (RR [95% CrI] = 0.02 [0, 0.28]; SUCRA: 90.76%), followed by vonoprazan (RR [95% CrI] = 0.03 [0, 0.19]; SUCRA: 86.47%), comparing with the placebo group. Pantoprazole also performed well in preventing GI hemorrhage (RR [95% CrI] = 0.01[0, 0.42]; SUCRA: 87.12%) compared with Teprenone. CONCLUSIONS: For patients with a history of PU receiving LDA, pantoprazole and vonoprazan might be the optimal choices to prevent PU recurrence and GI hemorrhage.
Recovery from indomethacin-induced gastrointestinal bleeding by treatment with teprenone.[Pubmed:38012767]
J Pharm Health Care Sci. 2023 Nov 28;9(1):44.
BACKGROUND: Gastrointestinal injuries caused by nonsteroidal anti-inflammatory drugs (NSAIDs) is a serious side effect in patients with rheumatoid arthritis (RA). However, effective therapeutic strategies have yet to be established. In this study, we investigated the therapeutic effects of Teprenone (TEP), a gastric mucosal protective drug, on NSAID-induced gastrointestinal injuries in rats with RA (AA rats). METHODS: Gastrointestinal injury was induced by oral administration of indomethacin (IMC), a typical NSAID. TEP was orally administered after IMC-induced gastrointestinal bleeding, and the stomach, jejunum, and ileum were excised. RESULTS: On day 14 of IMC administration, lesion areas in the stomach, jejunum, and ileum were significantly larger in AA rats than in normal rats. When TEP was orally administered to AA rats, the lesion areas in the stomach, jejunum, and ileum significantly decreased compared with those in control rats (IMC-induced AA rats). Therefore, we measured NOS2 mRNA and NO levels, which were significantly decreased in rats with IMC-induced AA after treatment with TEP. CONCLUSIONS: These results suggest that the oral administration of TEP may be useful for the treatment of NSAID-induced gastrointestinal injuries in patients with RA.
A randomized controlled trial of teprenone in terms of preventing worsening of COVID-19 infection.[Pubmed:37883347]
PLoS One. 2023 Oct 26;18(10):e0287501.
BACKGROUND: Some COVID-19 patients develop life-threatening disease accompanied by severe pneumonitis. Teprenone induces expression of heat-shock proteins (HSPs) that protect against interstitial pneumonia in preclinical models. We explored whether Teprenone prevented worsening of COVID-19 infections. METHODS: This open-label, randomized, pilot phase 2 clinical trial was conducted at five institutions in Japan. We randomized patients hospitalized for COVID-19 with fever to Teprenone or no-Teprenone groups in a 1:1 ratio. We stratified patients by sex, age < and >/= 70 years and the existence (or not) of complications (hypertension, diabetes, ischemic heart disease, chronic pulmonary disease and active cancer). No limitation was imposed on other COVID-19 treatments. The primary endpoint was the intubation rate. RESULTS: One hundred patients were included, 51 in the Teprenone and 49 in the no- Teprenone groups. The intubation rate did not differ significantly between the two groups: 9.8% (5/51) vs. 2.0% (1/49) (sub-hazard ratio [SHR] 4.99, 95% confidence interval [CI]: 0.59-42.1; p = 0.140). The rates of intra-hospital mortality and intensive care unit (ICU) admission did not differ significantly between the two groups: intra-hospital mortality 3.9% (2/51) vs. 4.1% (2/49) (hazard ratio [HR] 0.78, 95%CI: 0.11-5.62; p = 0.809); ICU admission 11.8% (6/51) vs. 6.1% (3/49) (SHR 1.99, 95%CI: 0.51-7.80; p = 0.325). CONCLUSION: Teprenone afforded no clinical benefit. TRIAL REGISTRATION: Japan Registry of Clinical Trials jRCTs061200002 (registered on 20/May/2020).
Endoscopic Submucosal Dissection and Teprenone for Early Gastric Cancer, With Evaluation of eCura Scoring System.[Pubmed:36947657]
Altern Ther Health Med. 2023 May;29(4):218-223.
CONTEXT: Early gastric cancer is a common, malignant, tumor disease. Compared with traditional surgical methods, endoscopic mucosal dissection (ESD) is a minimally invasive surgery; however, in practice, it still carries some surgical risks. Teprenone is a common drug that protects the gastric mucosa and promotes the recovery of gastric mucosal and gastrointestinal function. OBJECTIVE: The study intended to investigate the clinical efficacy of endoscopic mucosal dissection combined with Teprenone for early gastric cancer, including an evaluation of the combined treatment using the eCura scoring system, with a view to providing the results as a reference for the choice of treatment modality for early gastric cancer. DESIGN: The research team performed a prospective controlled study. SETTING: The study took place in the Department of General Surgery, Huidong, at Zigong Fourth People's Hospital in Zigong, China. PARTICIPANTS: Participants were patients with early gastric cancer, 58 who were admitted to the hospital between January 2019 and June 2020 and 58 who were admitted between July 2020 and December 2021. INTERVENTION: The research team assigned: (1) the 58 patients in the earlier group to the control group, and they received treatment using endoscopic mucosal dissection; and (2) the 58 patients in the latter group to be the intervention group, and they received treatment using endoscopic mucosal dissection combined with Teprenone. OUTCOME MEASURES: The research team examined participants' postoperative: (1) abdominal pain scores; (2) size of ulcer wound area, (3) complications-delayed bleeding, ulcer perforation, fever, or abdominal pain; (4) risk as measured by the eCura scoring system-low, medium, or high risk; and (5) survival rates of those assessed at different risks under the eCura scoring systems. RESULTS: Postoperatively, the intervention group's abdominal pain scores on days 3 and 5 and the size of the groups' ulcer areas at days 7 and 14 were significantly lower than those of the control group (all P < .001). The intervention group's total incidence of postoperative complications, at 3.45%, was significantly lower than that in the control group, at 20.69% (P = .004). The number of participants low risk was 39 (67.25%), as assessed by eCura scoring system, which was significantly higher than that of the control group, at 22 participants (37.93%). The intervention groups' overall survival rate, at 98.28%, was significantly higher than that of the control group, at 69.49% (P < .001). CONCLUSIONS: Endoscopic mucosal dissection combined with Teprenone as a treatment for early gastric cancer can achieve a significantly better therapeutic effect than can endoscopic mucosal dissection only. It can reduce the risk of postoperative complications and improve the assessment of risk found with the eCura scoring system. It can have an important role in improving the postoperative survival rate of patients with early gastric cancer and is worthy of clinical application.
Programmed Upregulation of HSP70 by Metal-Organic Frameworks Nanoamplifier for Enhanced Microwave Thermal-Immunotherapy.[Pubmed:36125400]
Adv Healthc Mater. 2022 Dec;11(23):e2201441.
Thermotherapy can directly kill tumor cells whilst being accompanied by immune-enhancing effects. However, this immune-enhancing effect suffers from insufficient expression of immune response factors (e.g., heat shock protein 70, HSP70), resulting in no patient benefiting due to the recurrence of tumor cells after thermotherapy. Herein, a nanoengineered strategy of programmed upregulating of the immune response factors for amplifying synergistic therapy is explored. Metal-organic frameworks nanoamplifiers (Teprenone/nitrocysteine@ZrMOF-NH(2) @L-menthol@triphenylphosphine, GGA/CSNO@ZrMOF-NH(2) -LM-TPP nanoamplifier, and GCZMT nanoamplifier) achieve excellent microwave (MW) thermal-immunotherapy by programmed induction of HSP70 expression. After intravenous administration, GCZMT nanoamplifiers target the mitochondria, and then release nitric oxide (NO) under MW irradiation. NO inhibits the growth of tumor cells by interfering with the energy supply of cells. Subsequently, under the combination of MW, NO, and GGA, HSP70 expression can be programmed upregulated, which can induce the response of cytotoxic CD4(+) T cells and CD8(+) T cells, and effectively activate antitumor immunotherapy. Hence, GCZMT nanoamplifier-mediated MW therapy can achieve a satisfactory therapeutic effect with the tumor inhibition of 97%. This research offers a distinctive insight into the exploitation of metal-organic frameworks nanoamplifiers for enhanced tumor therapy, which provides a new approach for highly effective cancer treatment.


