ZhebeirineCAS# 143120-47-2 |
- Delavinone
Catalog No.:BCX1554
CAS No.:96997-98-7
Quality Control & MSDS
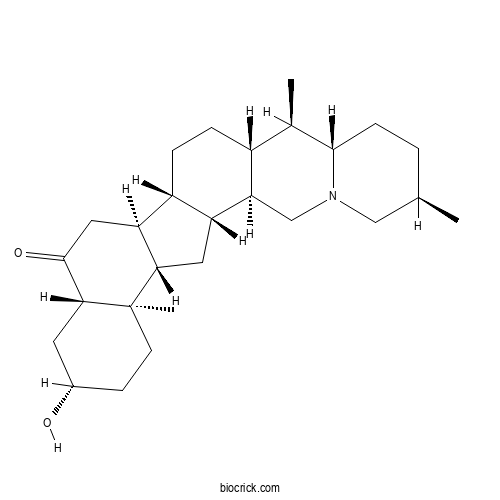
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 143120-47-2 | SDF | Download SDF |
| PubChem ID | 10597859.0 | Appearance | Powder |
| Formula | C27H43NO2 | M.Wt | 413.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2S,6R,9S,10R,11S,14S,15S,18S,20S,23R,24S)-20-hydroxy-6,10,23-trimethyl-4-azahexacyclo[12.11.0.02,11.04,9.015,24.018,23]pentacosan-17-one | ||
| SMILES | CC1CCC2C(C3CCC4C(C3CN2C1)CC5C4CC(=O)C6C5(CCC(C6)O)C)C | ||
| Standard InChIKey | MWBJDDYEYGDWCZ-PAYGNFRXSA-N | ||
| Standard InChI | InChI=1S/C27H43NO2/c1-15-4-7-25-16(2)18-5-6-19-20(22(18)14-28(25)13-15)11-23-21(19)12-26(30)24-10-17(29)8-9-27(23,24)3/h15-25,29H,4-14H2,1-3H3/t15-,16-,17+,18-,19-,20-,21+,22-,23+,24-,25+,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Zhebeirine Dilution Calculator

Zhebeirine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4175 mL | 12.0875 mL | 24.175 mL | 48.3501 mL | 60.4376 mL |
| 5 mM | 0.4835 mL | 2.4175 mL | 4.835 mL | 9.67 mL | 12.0875 mL |
| 10 mM | 0.2418 mL | 1.2088 mL | 2.4175 mL | 4.835 mL | 6.0438 mL |
| 50 mM | 0.0484 mL | 0.2418 mL | 0.4835 mL | 0.967 mL | 1.2088 mL |
| 100 mM | 0.0242 mL | 0.1209 mL | 0.2418 mL | 0.4835 mL | 0.6044 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Demethylwedelolactone sulfate
Catalog No.:BCX1463
CAS No.:1318240-80-0
- 6α-(3-methylvaleryloxy)-Britannilactone
Catalog No.:BCX1462
CAS No.:1260151-66-3
- 6α-isovaleryloxy-Britannilactone
Catalog No.:BCX1461
CAS No.:1259933-04-4
- 6α-isobutyryloxy-Britannilactone
Catalog No.:BCX1460
CAS No.:1259933-02-2
- Puerarin-4'-O-β-D-glucopyranoside
Catalog No.:BCX1459
CAS No.:117047-08-2
- 6α-(2-methybutyryloxy)-Britannilactone
Catalog No.:BCX1458
CAS No.:1260151-65-2
- Teprenone
Catalog No.:BCX1457
CAS No.:6809-52-5
- Fosinopril EP impurity D
Catalog No.:BCX1456
CAS No.:1356353-41-7
- Cirsiumaldehyde
Catalog No.:BCX1455
CAS No.:7389-38-0
- Linustatin
Catalog No.:BCX1454
CAS No.:72229-40-4
- Liraglutide
Catalog No.:BCX1453
CAS No.:204656-20-2
- 2'-Acetyltaxol
Catalog No.:BCX1452
CAS No.:92950-40-8
- Hordenine Chloride
Catalog No.:BCX1465
CAS No.:6027-23-2
- trans-Nerolidol
Catalog No.:BCX1466
CAS No.:40716-66-3
- Nor-β-anhydroicaritin
Catalog No.:BCX1467
CAS No.:28610-34-6
- Dehydroandrographolide Succinate Sodium Potasium Salt
Catalog No.:BCX1468
CAS No.:863319-40-8
- 4-Hydroxylonchocarpin
Catalog No.:BCX1469
CAS No.:56083-03-5
- Salicortin
Catalog No.:BCX1470
CAS No.:1887055-63-1
- Iodixanol Impurity E
Catalog No.:BCX1471
CAS No.:255376-57-9
- Ganoderenic acid C2
Catalog No.:BCX1472
CAS No.:1961358-00-8
- Ganoderic acid Gama
Catalog No.:BCX1473
CAS No.:294674-00-3
- Oleuropein Aglycone
Catalog No.:BCX1474
CAS No.:31773-95-2
- (20S)-Panaxatriol
Catalog No.:BCX1475
CAS No.:848830-68-2
- (20S)-Panaxadiol
Catalog No.:BCX1476
CAS No.:112791-34-1
Discovery of potential quality markers of Fritillariae thunbergii bulbus in pneumonia by combining UPLC-QTOF-MS, network pharmacology, and molecular docking.[Pubmed:36843054]
Mol Divers. 2023 Feb 26:1-18.
Fritillariae thunbergii bulbus (FTB) is a popular Chinese herbal medicine with various applications in respiratory diseases. The quality evaluation of FTB has been insufficient to date, as the active ingredients and mechanisms of action of FTB remain unclear. This study proposes a novel strategy for exploring the quality markers (Q-markers) of FTB based on UPLC-QTOF-MS analysis, network pharmacology, molecular docking, and molecular dynamics (MD) simulation. A total of 26 compounds in FTB were identified by UPLC-QTOF-MS. Ten of these compounds were screened as Q-markers based on network pharmacology for their anti-pneumonia effects, including imperialine, peimisine, peiminine, ebeiedinone, Zhebeirine, puqiedine, 9-hydroxy-10,12-octadecadienoic acid, (9Z,12Z,15Z)-13-hydroxy-9,12,15-octadecatrienoic acid, 9,12,15-octadecatrienoic acid, and (2E,4Z,7Z,10Z,13Z,16Z,19Z)-2,4,7,10,13,16,19-docosaheptaenoic acid methyl ester (DAME). These Q-markers were predicted to act on multiple targets and pathways associated with pneumonia. Molecular docking results revealed that most of the Q-markers showed high affinity with at least one of the main targets of pneumonia, and the top ten complexes were confirmed with MD simulation. Network pharmacology indicated that FTB may act on the TNF signaling pathway, HIF-1 signaling pathway, JAK-STAT signaling pathway, etc. The results demonstrated that imperialine (P8), peimisine (P9), peiminine (P11), ebeiedine (P15), Zhebeirine (P16), and puqiedine (P18) may be potential Q-markers of FTB, and AKT1, IL-6, VEGFA, TP53, EGFR, STAT3, PPARG, MMP9, and CASP3 may be promising therapeutic targets for pneumonia treatment that are worthy of further research.
Revealing active components and action mechanism of Fritillariae Bulbus against non-small cell lung cancer through spectrum-effect relationship and proteomics.[Pubmed:36587416]
Phytomedicine. 2023 Feb;110:154635.
BACKGROUND: Fritillariae Bulbus (FB) is widely used as a traditional medicine for the treatment of lung meridian diseases. It has been proved that FB has good anti-non-small cell lung cancer (NSCLC) activity. However, the active components and potential mechanism are still not clear. PURPOSE: To reveal the bioactive components of FB against NSCLC and potential mechanism through spectrum-effect relationship and proteomics. METHOD: First, the FB extract was chemically profiled by UHPLC-QTOF-MS and the inhibitory effect of FB extract on A549 cell viability was evaluated by Cell Counting Kit-8 assay. Second, orthogonal-partial least squares-regression analysis was applied to screen potential active compounds through correlating the chemical profile with corresponding inhibitory effect. Third, the anti-NSCLC activities of potential active components were further investigated in terms of cell proliferation, cell cycle and cell apoptosis in vitro and tumor growth in vivo. Finally, proteomics was utilized to reveal the underlying anti-NSCLC mechanism. RESULTS: Six potential active components including verticine, verticinone, Zhebeirine, ebeiedinone, yibeissine and peimisine were screened out by spectrum-effect relationship. Among them, Zhebeirine showed higher inhibitory effect on A549 cell viability with IC(50) value of 36.93 muM and dosage-dependent inhibition of A549 xenograft tumor growth in nude mice. Proteomics and western blotting assays indicated that Zhebeirine could arrest cell cycle by down-regulating the expressions of CDK1, CDK2, Cyclin A2, Cyclin B2 and inhibiting the phosphorylation of p53. Moreover, the proteins participating in p53 signaling pathway including PCNA, 14-3-3sigma, CHEK1 were significantly decreased, which suggested that Zhebeirine affected cell cycle progression through p53 signaling pathway. CONCLUSION: This study not only provides scientific evidence to support the clinical application of FB against NSCLC, but also demonstrates that Zhebeirine is a promising anti-NSCLC lead compound deserving further studies.
A new alkaloid with cytotoxic activity from Fritillaria thunbergii Miq.[Pubmed:34058935]
Nat Prod Res. 2022 Oct;36(20):5297-5303.
A new alkaloid named zhebeisine (1), together with four known compounds, eduardine (2), Zhebeirine (3), isoverticine (4), and verticine (5), was isolated from the bulbs of Fritillaria thunbergii Miq. The structure of the new compound was elucidated on the basis of extensive spectroscopic methods and the in vitro biological activities of it were evaluated as well. Compound 1 features a veratramine skeleton with a rare 6/6/5/6/6/6 fused-ring system, representing the first reported veratramine-type alkaloid with a new oxazinane ring (ring-F) in Fritillaria genus. The cytotoxic activities study revealed that compound 1 inhibited the cell proliferation of HT29 and DLD1 (IC(50) values of 25.1 and 48.8 microM, respectively) and also induced apoptosis of the above-mentioned two cancer cell lines.[Formula: see text].
An in-silico approach to identify the potential hot spots in SARS-CoV-2 spike RBD to block the interaction with ACE2 receptor.[Pubmed:33685364]
J Biomol Struct Dyn. 2022 Oct;40(16):7408-7423.
A novel acute viral pneumonia induced by SARS-CoV-2 exploded at the end of 2019, causing a severe medical and economic crisis. For developing specific pharmacotherapy against SARS-CoV-2, an in silico virtual screening was developed for the available in-house molecules. The conserved domain analysis was performed to identify the highly conserved and exposed amino acid regions in the SARS-CoV-2-S RBD sites. The Protein-Protein interaction analyses demonstrated the higher affinity between the SARS-CoV-2-S and ACE2 due to varieties of significant interactions between them. The computational alanine scanning mutation study has recognized the highly stabilized amino acids in the SARS-CoV-2-S RBD/ACE2 complex. The cumulative sequence investigations have inferred that Lys417, Phe486, Asn487, Tyr489, and Gln493 are perhaps the iconic target amino acids to develop a drug molecule or vaccine against SARS-CoV-2 infection. Most of the selected compounds include luteolin, Zhebeirine, 3-dehydroverticine, embelin, andrographolide, ophiopogonin D, crocin-1, sprengerinin A, B, C, peimine, etc. were exhibited distinguish drug actions through the strong hydrogen bonding with the hot spots of the RBD. Besides, the 100 ns molecular dynamics simulation and free energy binding analysis showed the significant efficacy of luteolin to inhibit the infection of SARS-CoV-2. Highlights:Highly conserved and exposed amino acids in the SARS-CoV-2-S-RBD sites has been identifiedComputational alanine scanning mutation study has recognized the highly stabilized hot spots in the SARS-CoV-2-S RBD/ACE2 complex.Virtual screening has been executed to identify the drug actions in the RBD regionMost of the selected natural products were involved in the distinctive strong interactions with hot spots of RBD to inhibit the infection of SARS-CoV-2.[Formula: see text] Communicated by Ramaswamy H. Sarma.


