LinustatinCAS# 72229-40-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

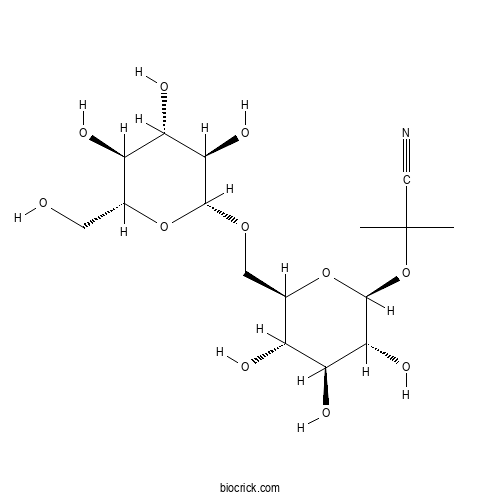
| Cas No. | 72229-40-4 | SDF | Download SDF |
| PubChem ID | 119301.0 | Appearance | Powder |
| Formula | C16H27NO11 | M.Wt | 409.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-methyl-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxypropanenitrile | ||
| SMILES | CC(C)(C#N)OC1C(C(C(C(O1)COC2C(C(C(C(O2)CO)O)O)O)O)O)O | ||
| Standard InChIKey | FERSMFQBWVBKQK-CXTTVELOSA-N | ||
| Standard InChI | InChI=1S/C16H27NO11/c1-16(2,5-17)28-15-13(24)11(22)9(20)7(27-15)4-25-14-12(23)10(21)8(19)6(3-18)26-14/h6-15,18-24H,3-4H2,1-2H3/t6-,7-,8-,9-,10+,11+,12-,13-,14-,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Linustatin Dilution Calculator

Linustatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4427 mL | 12.2133 mL | 24.4266 mL | 48.8532 mL | 61.0665 mL |
| 5 mM | 0.4885 mL | 2.4427 mL | 4.8853 mL | 9.7706 mL | 12.2133 mL |
| 10 mM | 0.2443 mL | 1.2213 mL | 2.4427 mL | 4.8853 mL | 6.1066 mL |
| 50 mM | 0.0489 mL | 0.2443 mL | 0.4885 mL | 0.9771 mL | 1.2213 mL |
| 100 mM | 0.0244 mL | 0.1221 mL | 0.2443 mL | 0.4885 mL | 0.6107 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Liraglutide
Catalog No.:BCX1453
CAS No.:204656-20-2
- 2'-Acetyltaxol
Catalog No.:BCX1452
CAS No.:92950-40-8
- Methyl cis-11-eicosenoate
Catalog No.:BCX1451
CAS No.:2390-09-2
- Stigmastanol
Catalog No.:BCX1450
CAS No.:83-45-4
- 16,17-Didehydroganoderic acid D
Catalog No.:BCX1449
CAS No.:1427189-02-3
- 4-Deoxyphorbol
Catalog No.:BCX1448
CAS No.:79083-67-3
- Neotame
Catalog No.:BCX1447
CAS No.:165450-17-9
- 6-Carboxyl-7-hydroxy-2,3-dimethylchromone
Catalog No.:BCX1446
CAS No.:108170-57-6
- 1β-Methoxydiversifolin
Catalog No.:BCX1445
CAS No.:110382-36-0
- Nicotinamide riboside
Catalog No.:BCX1444
CAS No.:1341-23-7
- Vernoflexuoside
Catalog No.:BCX1443
CAS No.:57576-33-7
- Rubrofusarin triglucoside
Catalog No.:BCX1442
CAS No.:245724-07-6
- Cirsiumaldehyde
Catalog No.:BCX1455
CAS No.:7389-38-0
- Fosinopril EP impurity D
Catalog No.:BCX1456
CAS No.:1356353-41-7
- Teprenone
Catalog No.:BCX1457
CAS No.:6809-52-5
- 6α-(2-methybutyryloxy)-Britannilactone
Catalog No.:BCX1458
CAS No.:1260151-65-2
- Puerarin-4'-O-β-D-glucopyranoside
Catalog No.:BCX1459
CAS No.:117047-08-2
- 6α-isobutyryloxy-Britannilactone
Catalog No.:BCX1460
CAS No.:1259933-02-2
- 6α-isovaleryloxy-Britannilactone
Catalog No.:BCX1461
CAS No.:1259933-04-4
- 6α-(3-methylvaleryloxy)-Britannilactone
Catalog No.:BCX1462
CAS No.:1260151-66-3
- Demethylwedelolactone sulfate
Catalog No.:BCX1463
CAS No.:1318240-80-0
- Zhebeirine
Catalog No.:BCX1464
CAS No.:143120-47-2
- Hordenine Chloride
Catalog No.:BCX1465
CAS No.:6027-23-2
- trans-Nerolidol
Catalog No.:BCX1466
CAS No.:40716-66-3
Profiling of Secondary Metabolites of Optimized Ripe Ajwa Date Pulp (Phoenix dactylifera L.) Using Response Surface Methodology and Artificial Neural Network.[Pubmed:37259461]
Pharmaceuticals (Basel). 2023 Feb 20;16(2):319.
The date palm (Phoenix dactylifera L.) is a popular edible fruit consumed all over the world and thought to cure several chronic diseases and afflictions. The profiling of the secondary metabolites of optimized ripe Ajwa date pulp (RADP) extracts is scarce. The aim of this study was to optimize the heat extraction (HE) of ripe Ajwa date pulp using response surface methodology (RSM) and artificial neural network (ANN) modeling to increase its polyphenolic content and antioxidant activity. A central composite design was used to optimize HE to achieve the maximum polyphenolic compounds and antioxidant activity of target responses as a function of ethanol concentration, extraction time, and extraction temperature. From RSM estimates, 75.00% ethanol and 3.7 h (extraction time), and 67 degrees C (extraction temperature) were the optimum conditions for generating total phenolic content (4.49 +/- 1.02 mgGAE/g), total flavonoid content (3.31 +/- 0.65 mgCAE/g), 2,2-diphenyl-1-picrylhydrazyl (11.10 +/- 0.78 % of inhibition), and cupric-reducing antioxidant capacity (1.43 microM ascorbic acid equivalent). The good performance of the ANN was validated using statistical metrics. Seventy-one secondary metabolites, including thirteen new bioactive chemicals (hebitol II, 1,2-di-(syringoyl)-hexoside, naringin dihydrochalcone, erythron-guaiacylglycerol-beta-syringaresinol ether hexoside, erythron-1-(4'-O-hexoside-3,5-dimethoxyphenyl)-2-syrngaresinoxyl-propane-1,3-diol, 2-deoxy-2,3-dehydro-N-acetyl-neuraminic acid, Linustatin and 1-deoxynojirimycin galactoside), were detected using high-resolution mass spectroscopy. The results revealed a significant concentration of phytoconstituents, making it an excellent contender for the pharmaceutical and food industries.
Application of a comprehensive metabolomics approach for the selection of flaxseed varieties with the highest nutritional and medicinal attributes.[Pubmed:35696216]
J Food Drug Anal. 2021 Jun 15;29(2):214-239.
Flaxseed is considered an indispensable generally recognized as safe (GRAS) source of polyphenolic lignans, polyunsaturated fatty acids (PUFA), fibers as well as minerals and vitamins. The metabolite content of flaxseed reflects its nutritional and medicinal value. Therefore, the selection of flaxseed variety for food industry is dependent on its metabolome. A metabolomics approach based on liquid or gas chromatography coupled to mass spectrometry has been applied to discriminate different flaxseed cultivars that are commercially available in Egypt. The available Sakha cultivars were subjected to a comprehensive metabolomics and lipidomics approach for investigation of their metabolomes. Our results showed that among the screened cultivars, Sakha 6, with its yellow-colored testa, showed marked metabolic discrimination. This yellow cultivar showed high accumulation of essential amino acids. Additionally, the oil of this cultivar accumulated the highest content of the two essential PUFA: alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid). Interestingly, the content of the main antinutritional cyanogenic glycosides such as Linustatin and neoLinustatin was lower, while, the content of medicinally-important secondary metabolites was higher in Sakha 6 cultivar. These results support the use of this cultivar for human consumption owing to its high nutritional and medicinal value.
Metabolism of the Cyanogenic Glucosides in Developing Flax: Metabolic Analysis, and Expression Pattern of Genes.[Pubmed:32674262]
Metabolites. 2020 Jul 14;10(7):288.
Cyanogenic glucosides (CG), the monoglycosides linamarin and lotaustralin, as well as the diglucosides Linustatin and neoLinustatin, have been identified in flax. The roles of CG and hydrogen cyanide (HCN), specifically the product of their breakdown, differ and are understood only to a certain extent. HCN is toxic to aerobic organisms as a respiratory inhibitor and to enzymes containing heavy metals. On the other hand, CG and HCN are important factors in the plant defense system against herbivores, insects and pathogens. In this study, fluctuations in CG levels during flax growth and development (using UPLC) and the expression of genes encoding key enzymes for their metabolism (valine N-monooxygenase, linamarase, cyanoalanine nitrilase and cyanoalanine synthase) using RT-PCR were analyzed. Linola cultivar and transgenic plants characterized by increased levels of sulfur amino acids were analyzed. This enabled the demonstration of a significant relationship between the cyanide detoxification process and general metabolism. Cyanogenic glucosides are used as nitrogen-containing precursors for the synthesis of amino acids, proteins and amines. Therefore, they not only perform protective functions against herbivores but are general plant growth regulators, especially since changes in their level have been shown to be strongly correlated with significant stages of plant development.
Development and validation of eight cyanogenic glucosides via ultra-high-performance liquid chromatography-tandem mass spectrometry in agri-food.[Pubmed:32593038]
Food Chem. 2020 Nov 30;331:127305.
An ultra-high-performance liquid chromatography-triple quadrupole tandem mass spectrometry (UHPLC-QqQ-MS/MS) method was established and validated for the simultaneous quantification of eight cyanogenic glucosides (CNGs) in agri-food. The eight CNGs were linamarin, lotaustralin, Linustatin, neoLinustatin, taxiphyllin, amygdalin, dhurrin and prunasin. CNGs were extracted with aqueous methanol and cleaned via solid-phase extraction. Analytes were separated with a C(18) column via gradient elution. MS/MS analysis was performed with electrospray ionisation in positive mode. Quantification was performed in multiple reaction monitoring mode. Satisfactory validation results were obtained in terms of linearity, sensitivity, precision and accuracy, matrix effect and stability. The method was applied in typical cyanogenic agri-food. CNGs in cassava, linseed, bamboo, sorghum, apricot, almond and lima bean were analyzed.
Development and validation of an ultra-high performance liquid chromatography-high resolution mass spectrometry method for simultaneous quantification of cyanogenic glycosides and secoisolariciresinol diglucoside in flaxseed (Linum usitatissimum L.).[Pubmed:31076224]
J Chromatogr A. 2019 Sep 13;1601:214-223.
An ultra performance liquid chromatography electrospray ionization high-resolution mass spectrometry (UPLC/ESI-HRMS) method was developed and validated for simultaneous quantification of cyanogenic glycosides (CGs), [Linustatin (LIS) and neoLinustatin (NLIS)], and the main lignan, secoisolariciresinol diglucoside (SDG) in Linoforce(R) (LF) [flaxseed (Linum usitatissimum L.) coated with two herbal extracts (Senna alexandrina mill and Frangula alnus)]. CGs and SDG were extracted from defatted ground LF by a new procedure consisting of an aqueous methanol ultrasound-assisted extraction followed by an aqueous alkaline ultrasound-assisted extraction of the residue. The combined extracted solutions were then hydrolyzed by 0.02 M NaOH to release SDG from its hydroxymethyl glutaryl ester-linked complex (SDG-HMG). After hydrolysis, the sample was acidified and analyzed directly, without the need of any additional clean-up steps, by UPLC/ESI-HRMS in positive mode. The identification of CGs and SDG was confirmed by the similar retention time and similar MS spectra to the corresponding authentic standards. The quantification was performed using the corresponding extracted ion chromatograms and amygdalin as internal standard. The overall method was validated in terms of linearity, stability, selectivity, precision and accuracy. The developed method was successfully applied to the quantification of CGs and SDG in LF and also in non-coated flaxseed. This is the first report on the simultaneous quantification of CGs and SDG in LF and flaxseed.
Metabolism of cyanogenic glycosides: A review.[Pubmed:30615957]
Food Chem Toxicol. 2019 Mar;125:225-232.
Potential toxicity of cyanogenic glycosides arises from enzymatic degradation to produce hydrogen cyanide. Information on the metabolism of cyanogenic glycosides is available from in vitro, animal and human studies. In the absence of beta-glucosidase enzymes from the source plant material, two processes appear to contribute to the production of cyanide from cyanogenic glycosides; the proportion of the glycoside dose that reaches the large intestine, where most of the bacterial hydrolysis occurs, and the rate of hydrolysis of cyanogenic glycosides to cyanohydrin and cyanide. Some cyanogenic glycosides, such as prunasin, are actively absorbed in the jejunum by utilising the epithelial sodium-dependent monosaccharide transporter (SGLT1). The rate of cyanide production from cyanogenic glycosides due to bacterial beta-glycosidase activity depends on; the sugar moiety in the molecule and the stability of the intermediate cyanohydrin following hydrolysis by bacterial beta-glucosidase. Cyanogenic glycosides with a gentiobiose sugar, amygdalin, Linustatin, and neoLinustatin, undergo a two stage hydrolysis, with gentiobiose initially being hydrolysed to glucose to form prunasin, linamarin and lotaustralin, respectively. While the overall impact of these metabolic factors is difficult to predict, the toxicity of cyanogenic glycosides will be less than the toxicity suggested by their theoretical hydrocyanic acid equivalents.
Use of qNMR for speciation of flaxseeds (Linum usitatissimum) and quantification of cyanogenic glycosides.[Pubmed:29116353]
Anal Bioanal Chem. 2017 Dec;409(30):7011-7026.
This report describes a routine method taking less than 20 min to quantify cyanogenic glycosides such as Linustatin and neoLinustatin from flaxseeds (Linum usitatissimum L.) using (1)H nuclear magnetic resonance. After manual dehulling, a higher Linustatin content was shown in the almond fraction, while neoLinustatin and total cyanogenic glycoside contents were significantly higher in hulls. Linustatin and neoLinustatin were quantified in seven cultivars grown in two locations in three different years. Linustatin, neoLinustatin, and total cyanogenic glycosides ranged between 91 and 267 mg/100 g, 78-272 mg/100 g, and 198-513 mg/100 g dry weight flaxseeds, respectively. NMR revealed differences of up to 70% between samples with standard deviation variations lower than 6%. This study shows that NMR is a very suitable tool to perform flaxseed varietal selection for the cyanogenic glycoside content. Graphical abstract qNMR can be used to perform flaxseed varietal selection for the cyanogenic glycoside content.
Secoisolariciresinol Diglucoside and Cyanogenic Glycosides in Gluten-free Bread Fortified with Flaxseed Meal.[Pubmed:27998066]
J Agric Food Chem. 2016 Dec 21;64(50):9551-9558.
Flaxseed (Linum usitatissimum L.) meal contains cyanogenic glycosides (CGs) and the lignan secoisolariciresinol diglucoside (1). Gluten-free (GF) doughs and baked goods were produced with added flaxseed meal (20%, w/w) then 1, and CGs were determined in fortified flour, dough, and bread with storage (0, 1, 2, and 4 weeks) at different temperatures (-18, 4, and 22-23 degrees C). 1 was present in flour, dough, and GF bread after baking. 1 was stable with extensive storage (up to 4 weeks) and was not affected by storage temperature. CGs in flaxseed meal and fortified GF samples were analyzed by (1)H NMR of the cyanohydrins. Linamarin and/or Linustatin were the primary CGs in both flaxseed meal and fortified flour. CGs decreased with storage in dough fortified with flaxseed meal or GF bread after baking. GF bakery food products fortified with flaxseed meal had reduced CGs but remained a good source of dietary 1.
Cyanogenetic glycosides and simple glycosides from the linseed meal.[Pubmed:26307006]
Fitoterapia. 2015 Oct;106:78-83.
Three new cyanogenetic triglycosides Linustatins A-C (1-3), and two new simple glycosides Linustatins D and E (4 and 5) were isolated from the 70% ethanol extract of flaxseed meal (Linum usitatissimum L.). Their structures were elucidated on the basis of spectroscopic analysis and chemical evidence. All of the isolates showed moderate activities against aldose reductase and weak activities against alpha-glucosidase, DPP-IV, and FBPase at the same concentrations as the positive control drugs.
Dirigent Protein-Mediated Lignan and Cyanogenic Glucoside Formation in Flax Seed: Integrated Omics and MALDI Mass Spectrometry Imaging.[Pubmed:25981198]
J Nat Prod. 2015 Jun 26;78(6):1231-42.
An integrated omics approach using genomics, transcriptomics, metabolomics (MALDI mass spectrometry imaging, MSI), and bioinformatics was employed to study spatiotemporal formation and deposition of health-protecting polymeric lignans and plant defense cyanogenic glucosides. Intact flax (Linum usitatissimum) capsules and seed tissues at different development stages were analyzed. Transcriptome analyses indicated distinct expression patterns of dirigent protein (DP) gene family members encoding (-)- and (+)-pinoresinol-forming DPs and their associated downstream metabolic processes, respectively, with the former expressed at early seed coat development stages. Genes encoding (+)-pinoresinol-forming DPs were, in contrast, expressed at later development stages. Recombinant DP expression and DP assays also unequivocally established their distinct stereoselective biochemical functions. Using MALDI MSI and ion mobility separation analyses, the pinoresinol downstream derivatives, secoisolariciresinol diglucoside (SDG) and SDG hydroxymethylglutaryl ester, were localized and detectable only in early seed coat development stages. SDG derivatives were then converted into higher molecular weight phenolics during seed coat maturation. By contrast, the plant defense cyanogenic glucosides, the monoglucosides linamarin/lotaustralin, were detected throughout the flax capsule, whereas diglucosides Linustatin/neoLinustatin only accumulated in endosperm and embryo tissues. A putative biosynthetic pathway to the cyanogens is proposed on the basis of transcriptome coexpression data. Localization of all metabolites was at ca. 20 mum resolution, with the web based tool OpenMSI enabling not only resolution enhancement but also an interactive system for real-time searching for any ion in the tissue under analysis.
[Chemical constituents from the linseed meal].[Pubmed:23833939]
Yao Xue Xue Bao. 2013 Apr;48(4):521-5.
Ten compounds were isolated from the 70% ethanol extract of linseed meal (Linum usitatissimum L) through a combination of various chromatographic techniques, including silica gel, macroporous adsorbent resin, Sephadex LH-20, and preparative HPLC. On the basis of spectroscopic data analysis, they were elucidated as 1-methylethyl-2-O-beta-D-glucopyranosyl-(1" --> 6')-beta-D-glucopyanoside (1), Linustatin (2), neoLinustatin (3), lotaustralin (4), linamarin (5), deoxyguanosine (6), deoxyadenosine (7), (+)-pinoresinol-4'-O-beta-D-glucopyranoside (8), 4-O-beta-D-glucopyranosylvanillyl alcohol (9) and tachioside (10), separately. Among them, compound 1 is a new compound, and compounds 6, 8 and 10 were isolated from the linseed meal for the first time.
Development of optimized extraction methodology for cyanogenic glycosides from flaxseed (Linum usitatissimum).[Pubmed:20480892]
J AOAC Int. 2010 Mar-Apr;93(2):478-84.
A reference method (higher accuracy) and a routine method (higher throughput) were developed for the extraction of cyanogenic glycosides from flaxseed. Conditions of (essentially) complete extraction were identified by comparing grinding methods and extraction solvent composition, and optimizing solvent-to-meal ratio, extraction time, and repeat extraction. The reference extraction method consists of sample grinding using a high-speed impact plus sieving mill at 18 000 rpm with a 1.0 mm sieve coupled with triple-pooled extraction in a sonicating water bath (40 degrees C, 30 min) using 75% methanol. The routine method differs by the use of a coffee mill to grind samples and a single extraction. The 70 and 80% methanol solutions were equal and superior to other combinations from 50 to 100% aqueous ethanol or methanol. The extraction efficiencies of the routine method (relative to the reference method) was 87.9 +/- 2.0% SD (Linustatin) and 87.6 +/- 1.9% SD (neoLinustatin) using four composite samples that were generated from seeds of multiple cultivars over two crop years and locations across Western Canada. Ground flaxseed was stable after storage at room temperature, refrigeration, or freezing for up to 7 days, and frozen for at least 2 weeks but less than 2 months. Extracts were stable for up to 1 week at room temperature and at least 2 weeks when refrigerated or frozen.
The leaf, inner bark and latex cyanide potential of Hevea brasiliensis: evidence for involvement of cyanogenic glucosides in rubber yield.[Pubmed:19409582]
Phytochemistry. 2009 Apr;70(6):730-9.
The latex of Hevea brasiliensis, expelled upon bark tapping, is the cytoplasm of anastomosed latex cells in the inner bark of the rubber tree. Latex regeneration between two tappings is one of the major limiting factors of rubber yield. Hevea species contain high amounts of cyanogenic glucosides from which cyanide is released when the plant is damaged providing an efficient defense mechanism against herbivores. In H. brasiliensis, the cyanogenic glucosides mainly consist of the monoglucoside linamarin (synthesized in the leaves), and its diglucoside transport-form, Linustatin. Variations in leaf cyanide potential (CNp) were studied using various parameters. Results showed that the younger the leaf, the higher the CNp. Leaf CNp greatly decreased when leaves were directly exposed to sunlight. These results allowed us to determine the best leaf sampling conditions for the comparison of leaf CNp. Under these conditions, leaf CNp was found to vary from less than 25 mM to more than 60 mM. The rubber clones containing the highest leaf CNp were those with the highest yield potential. In mature virgin trees, the CNp of the trunk inner bark was shown to be proportional to leaf CNp and to decrease on tapping. However, the latex itself exhibited very low (if any) CNp, while harboring all the enzymes (beta-D-diglucosidase, linamarase and beta-cyanoalanine synthase) necessary to metabolize cyanogenic glucosides to generate non-cyanogenic compounds, such as asparagine. This suggests that in the rubber tree bark, cyanogenic glucosides may be a source of buffering nitrogen and glucose, thereby contributing to latex regeneration/production.
Development of extraction and gas chromatography analytical methodology for cyanogenic glycosides in flaxseed (Linum usitatissimum).[Pubmed:17373446]
J AOAC Int. 2007 Jan-Feb;90(1):153-61.
The development of well-characterized rapid methodology for the extraction and gas chromatographic analysis of the cyanogenic glycosides Linustatin and neoLinustatin from flaxseed (Linum usitatissimum L.) is reported. Two quantitation methods using phenyl-beta-D-glucopyranoside as an internal standard are described: direct quantitation using Linustatin and neoLinustatin external standard curves [standard curve slope variabilities of 2.6 and 5.7% relative standard deviation (RSD), respectively, over 7 days] or by use of methyl-alpha-D-glucopyranoside as a surrogate external standard, with conversion factors to convert to Linustatin and neoLinustatin concentration [1.109 +/- 0.015 (SD) mg Linustatin/mg methyl-alpha-D-glucopyranoside and 1.180 +/-0.067 (SD) mg neoLinustatin/mg methyl-alpha-D-glucopyranoside]. The former method is direct, thereby contributing less uncertainty to the method, and the latter adds a small degree of uncertainty coupled with considerable cost savings. Limits of detection for all standards were in the low- to sub-nanogram level and were 10-100 times lower than the lower limit of quantitation (LOQ). Repeatability precision was performed on 2 separate days at the lower and upper LOQs, with the RSD in peak response being 1% or lower in all cases. Extraction methods were evaluated for their ability to extract Linustatin and neoLinustatin from flaxseed using several combinations of aqueous ethanol, and recoveries were determined against the highest yielding method. Recoveries were as low as 82%, indicating that optimized extraction methodology is critical for the accuracy of results.
Raman spectroscopic analysis of cyanogenic glucosides in plants: development of a flow injection surface-enhanced Raman scatter (FI-SERS) method for determination of cyanide.[Pubmed:15000716]
Appl Spectrosc. 2004 Feb;58(2):212-7.
Cyanogenic glucosides were studied using Raman spectroscopy. Spectra of the crystal forms of linamarin, Linustatin, neoLinustatin, amygdalin, sambunigrin, and dhurrin were obtained using a Raman spectrograph microscope equipped with a 532 nm laser. The position of the signal from the C identical with N triple bond of the cyanohydrin group was influenced by the nature of the side group and was above 2240 cm(-1) for the three cyanogenic glucosides that contain a neighboring aromatic ring, and below or partially below 2240 cm(-1) for the non-aromatic cyanoglucosides. Signals from the CN bond of linamarin/lotaustralin in leaves and roots from a medium cyanogenic cassava variety were obtained in situ using a Fourier transform near-infrared (FT-NIR) Raman interferometer with a 1064 nm laser, but the signal was very weak and difficult to obtain. A spectrum containing a signal from the CN bond of dhurrin in a freeze-dried sorghum leaf was also obtained using this instrument. Surface-enhanced Raman Spectroscopy (SERS) was demonstrated to be a more sensitive method that enabled determination of the cyanogenic potential of plant tissue. The SERS method was optimized by flow injection (FI) using a colloidal gold dispersion as effluent. Potential problems and pitfalls of the method are discussed.


