Nicotinamide ribosideCAS# 1341-23-7 |
Quality Control & MSDS
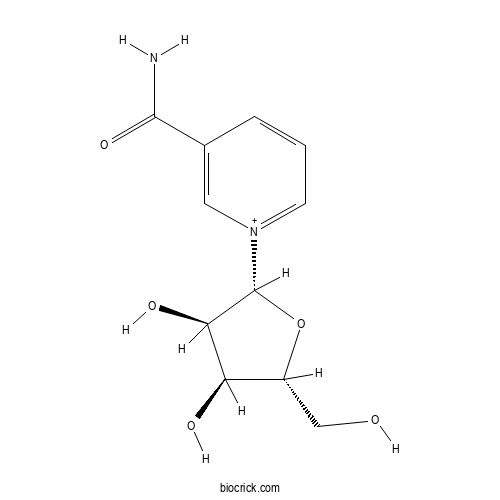
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1341-23-7 | SDF | Download SDF |
| PubChem ID | 439924.0 | Appearance | Powder |
| Formula | C11H15N2O5+ | M.Wt | 255.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyridin-1-ium-3-carboxamide | ||
| SMILES | C1=CC(=C[N+](=C1)C2C(C(C(O2)CO)O)O)C(=O)N | ||
| Standard InChIKey | JLEBZPBDRKPWTD-TURQNECASA-O | ||
| Standard InChI | InChI=1S/C11H14N2O5/c12-10(17)6-2-1-3-13(4-6)11-9(16)8(15)7(5-14)18-11/h1-4,7-9,11,14-16H,5H2,(H-,12,17)/p+1/t7-,8-,9-,11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Nicotinamide riboside Dilution Calculator

Nicotinamide riboside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9177 mL | 19.5886 mL | 39.1773 mL | 78.3546 mL | 97.9432 mL |
| 5 mM | 0.7835 mL | 3.9177 mL | 7.8355 mL | 15.6709 mL | 19.5886 mL |
| 10 mM | 0.3918 mL | 1.9589 mL | 3.9177 mL | 7.8355 mL | 9.7943 mL |
| 50 mM | 0.0784 mL | 0.3918 mL | 0.7835 mL | 1.5671 mL | 1.9589 mL |
| 100 mM | 0.0392 mL | 0.1959 mL | 0.3918 mL | 0.7835 mL | 0.9794 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Vernoflexuoside
Catalog No.:BCX1443
CAS No.:57576-33-7
- Rubrofusarin triglucoside
Catalog No.:BCX1442
CAS No.:245724-07-6
- Tagitinin C
Catalog No.:BCX1441
CAS No.:59979-56-5
- 3'-Hydroxyflavanone
Catalog No.:BCX1440
CAS No.:92496-65-6
- D-mannuronic acid sodium salt
Catalog No.:BCX1439
CAS No.:921-56-2
- D-dimannuronic acid disodium salt
Catalog No.:BCX1438
CAS No.:34044-53-6
- D-trimannuronic acid trisodium salt
Catalog No.:BCX1437
CAS No.:66754-13-0
- D-tetramannuronic acid tetrasodium salt
Catalog No.:BCX1436
CAS No.:149511-34-2
- D-pentamannuronic acid pentasodium salt
Catalog No.:BCX1435
CAS No.:183668-50-0
- D-hexamannuronic acid hexasodium salt
Catalog No.:BCX1434
CAS No.:183668-52-2
- D-heptamannuronic acid heptasodium salt
Catalog No.:BCX1433
CAS No.:862694-97-1
- D-octamannuronic acid octasodium salt
Catalog No.:BCX1432
CAS No.:862694-98-2
- 1β-Methoxydiversifolin
Catalog No.:BCX1445
CAS No.:110382-36-0
- 6-Carboxyl-7-hydroxy-2,3-dimethylchromone
Catalog No.:BCX1446
CAS No.:108170-57-6
- Neotame
Catalog No.:BCX1447
CAS No.:165450-17-9
- 4-Deoxyphorbol
Catalog No.:BCX1448
CAS No.:79083-67-3
- 16,17-Didehydroganoderic acid D
Catalog No.:BCX1449
CAS No.:1427189-02-3
- Stigmastanol
Catalog No.:BCX1450
CAS No.:83-45-4
- Methyl cis-11-eicosenoate
Catalog No.:BCX1451
CAS No.:2390-09-2
- 2'-Acetyltaxol
Catalog No.:BCX1452
CAS No.:92950-40-8
- Liraglutide
Catalog No.:BCX1453
CAS No.:204656-20-2
- Linustatin
Catalog No.:BCX1454
CAS No.:72229-40-4
- Cirsiumaldehyde
Catalog No.:BCX1455
CAS No.:7389-38-0
- Fosinopril EP impurity D
Catalog No.:BCX1456
CAS No.:1356353-41-7
Nicotinamide Mononucleotide and Nicotinamide Riboside Reverse Ovarian Aging in Rats Via Rebalancing Mitochondrial Fission and Fusion Mechanisms.[Pubmed:38684562]
Pharm Res. 2024 Apr 29.
PURPOSE: This study examined the effects of nicotinamide mononucleotide (NMN) and Nicotinamide riboside (NR) on folliculogenesis and mitochondrial dynamics (fission and fusion mechanisms) in ovaries of middle-aged female rats. METHODS: Experimental groups were young, middle-aged (control), middle-aged + NMN and middle-aged + NR. NMN was administered at a concentration of 500 mg/kg intraperitoneally but NR at a concentration of 200 mg/kg by gavage. Follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels were analyzed by ELISA. Hematoxylin-eosin staining sections were used for histopathological examination and follicles-counting. Expression levels of mitochondrial fission (Drp1, Mff and Fis1) and fusion (Mfn1, Mfn2, Opa1, Fam73a and Fam73b) genes as well as Sirt1 gene were analyzed by RT-PCR. Expression levels of fission-related proteins (DRP1, MFF, FIS1 and SIRT1) were analyzed by Western Blot. RESULTS: Higher ovarian index, more corpus luteum and antral follicles were detected in NMN and NR groups compared to the control. NMN or NR could rebalance LH/FSH ratio. The control group was determined to possess higher expression levels of fission genes and lower expression levels of fusion genes when compared the young group. In comparison with the control group, both NMN and NR group were found to exhibit less mitochondrial fission but more mitochondrial fussion. Higher gene and protein levels for Sirt1 were measured in NMN and NR groups compared to the control group. CONCLUSION: This study reveals that NMN alone or NR alone can rebalance mitochondrial dynamics by decreasing excessive fission in middle-aged rat ovaries, thus alleviating mitochondrial stress and correcting aging-induced folliculogenesis abnormalities.
Inhibition of UVB radiation-induced tissue swelling and immune suppression by nicotinamide riboside and pterostilbene.[Pubmed:38676310]
Photodermatol Photoimmunol Photomed. 2024 May;40(3):e12961.
BACKGROUND: Environmental ultraviolet radiation has deleterious effects on humans, including sunburn and immune perturbations. These immune changes are involved in skin carcinogenesis. OBJECTIVES: To determine whether Nicotinamide riboside and/or pterostilbene administered systemically inhibits inflammatory and immune effects of exposure to mid-range ultraviolet radiation. METHODS: To examine UVB radiation-induced inflammatory effects, mice were fed standard chow/water, 0.04% pterostilbene in chow and 0.2% Nicotinamide riboside in drinking water, diet with Nicotinamide riboside alone, or diet with pterostilbene alone. After 4 weeks, mice were exposed to UVB radiation (3500 J/m(2)), and 24-/48-h ear swelling was assessed. We also asked if each agent or the combination inhibits UVB radiation suppression of contact hypersensitivity in two models. Mice were fed standard diet/water or chow containing 0.08% pterostilbene, water with 0.4% Nicotinamide riboside, or both for 4 weeks. Low-dose: Half the mice in each group were exposed on the depilated dorsum to UVB radiation (1700 J/m(2)) daily for 4 days, whereas half were mock-irradiated. Mice were immunized on the exposed dorsum to dinitrofluorobenzene 4 h after the last irradiation, challenged 7 days later on the ears with dinitrofluorobenzene, and 24-h ear swelling assessed. High dose: Mice were treated similarly except that a single dose of 10,000 J/m(2) of radiation was administered and immunization was performed on the unirradiated shaved abdomen 3 days later. RESULTS: Nicotinamide riboside and pterostilbene together inhibited UVB-induced skin swelling more than either alone. Pterostilbene alone and both given together could inhibit UVB-induced immune suppression in both the low-dose and high-dose models while Nicotinamide riboside alone was more effective in the low-dose model than the high-dose model. CONCLUSION: Nicotinamide riboside and pterostilbene have protective effects against UVB radiation-induced tissue swelling and immune suppression.
Combined Metabolic Activators with Different NAD+ Precursors Improve Metabolic Functions in the Animal Models of Neurodegenerative Diseases.[Pubmed:38672280]
Biomedicines. 2024 Apr 22;12(4):927.
BACKGROUND: Mitochondrial dysfunction and metabolic abnormalities are acknowledged as significant factors in the onset of neurodegenerative disorders such as Parkinson's disease (PD) and Alzheimer's disease (AD). Our research has demonstrated that the use of combined metabolic activators (CMA) may alleviate metabolic dysfunctions and stimulate mitochondrial metabolism. Therefore, the use of CMA could potentially be an effective therapeutic strategy to slow down or halt the progression of PD and AD. CMAs include substances such as the glutathione precursors (L-serine and N-acetyl cysteine), the NAD+ precursor (Nicotinamide riboside), and L-carnitine tartrate. METHODS: Here, we tested the effect of two different formulations, including CMA1 (Nicotinamide riboside, L-serine, N-acetyl cysteine, L-carnitine tartrate), and CMA2 (nicotinamide, L-serine, N-acetyl cysteine, L-carnitine tartrate), as well as their individual components, on the animal models of AD and PD. We assessed the brain and liver tissues for pathological changes and immunohistochemical markers. Additionally, in the case of PD, we performed behavioral tests and measured responses to apomorphine-induced rotations. FINDINGS: Histological analysis showed that the administration of both CMA1 and CMA2 formulations led to improvements in hyperemia, degeneration, and necrosis in neurons for both AD and PD models. Moreover, the administration of CMA2 showed a superior effect compared to CMA1. This was further corroborated by immunohistochemical data, which indicated a reduction in immunoreactivity in the neurons. Additionally, notable metabolic enhancements in liver tissues were observed using both formulations. In PD rat models, the administration of both formulations positively influenced the behavioral functions of the animals. INTERPRETATION: Our findings suggest that the administration of both CMA1 and CMA2 markedly enhanced metabolic and behavioral outcomes, aligning with neuro-histological observations. These findings underscore the promise of CMA2 administration as an effective therapeutic strategy for enhancing metabolic parameters and cognitive function in AD and PD patients.
The Effectiveness of Four Nicotinamide Adenine Dinucleotide (NAD(+)) Precursors in Alleviating the High-Glucose-Induced Damage to Hepatocytes in Megalobrama amblycephala: Evidence in NAD(+) Homeostasis, Sirt1/3 Activation, Redox Defense, Inflammatory Response, Apoptosis, and Glucose Metabolism.[Pubmed:38671834]
Antioxidants (Basel). 2024 Mar 22;13(4):385.
The administration of NAD(+) precursors is a potential approach to protect against liver damage and metabolic dysfunction. However, the effectiveness of different NAD(+) precursors in alleviating metabolic disorders is still poorly elucidated. The current study was performed to compare the effectiveness of four different NAD(+) precursors, including nicotinic acid (NA), niacinamide (NAM), Nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN) in alleviating high-glucose-induced injury to hepatocytes in a fish model, Megalobrama amblycephala. An in vitro high-glucose model was successfully established to mimic hyperglycemia-induced damage to the liver, which was evidenced by the reduced cell viability, the increased transaminase activity, and the depletion of cellular NAD(+) concentration. The NAD(+) precursors all improved cell viability, with the maximal effect observed in NR, which also had the most potent NAD(+) boosting capacity and a significant Sirt1/3 activation effect. Meanwhile, NR presented distinct and superior effects in terms of anti-oxidative stress, inflammation inhibition, and anti-apoptosis compared with NA, NAM, and NMN. Furthermore, NR could effectively benefit glucose metabolism by activating glucose transportation, glycolysis, glycogen synthesis and the pentose phosphate pathway, as well as inhibiting gluconeogenesis. Moreover, an oral gavage test confirmed that NR presented the most potent effect in increasing hepatic NAD(+) content and the NAD(+)/NADH ratio among four NAD(+) precursors. Together, the present study results demonstrated that NR is most effective in attenuating the high-glucose-induced injury to hepatocytes in fish compared to other NAD(+) precursors.
A Magnesium Binding Site And The Anomeric Effect Regulate The Abiotic Redox Chemistry Of Nicotinamide Nucleotides.[Pubmed:38640109]
Chemistry. 2024 Apr 19:e202400411.
Nicotinamide adenine dinucleotide (NAD+) is a redox active molecule that is universally found in biology. Despite the importance and simplicity of this molecule, few reports exist that investigate which molecular features are important for the activity of this ribodinucleotide. By exploiting the nonenzymatic reduction and oxidation of NAD+ by pyruvate and methylene blue, respectively, we were able to identify key molecular features necessary for the intrinsic activity of NAD+ through kinetic analysis. Such features may explain how NAD+ could have been selected early during the emergence of life. Simpler molecules, such as nicotinamide, that lack an anomeric carbon are incapable of accepting electrons from pyruvate. The phosphate moiety inhibits activity in the absence of metal ions but facilitates activity at physiological pH and model prebiotic conditions by recruiting catalytic Mg2+. Reduction proceeds through consecutive single electron transfer events. Of the derivatives tested, including nicotinamide mononucleotide, Nicotinamide riboside, 3-(aminocarbonyl)-1-(2,3-dihydroxypropyl)pyridinium, 1-methylnicotinamide, and nicotinamide, only NAD+ and nicotinamide mononucleotide would be capable of efficiently accepting and donating electrons from and to pyruvate within a nonenzymatic electron transport chain. The data are consistent with early metabolic chemistry exploiting NAD+ or nicotinamide mononucleotide and not simpler molecules.
Boosting wound healing in diabetic rats: The role of nicotinamide riboside and resveratrol in UPR modulation and pyroptosis inhibition.[Pubmed:38583241]
Int Immunopharmacol. 2024 May 10;132:112013.
BACKGROUND: Diabetes-related skin ulcers provide a substantial therapeutic issue, sometimes leading to amputation, needing immediate practical treatments for efficient wound care. While the exact mechanisms are unknown, pyroptosis and deregulation of the unfolded protein response (UPR) are known to exacerbate inflammation. Nicotinamide riboside (NR) and Resveratrol (RV), which are known for their Nicotinamide adenine dinucleotide (NAD(+)) boosting and anti-inflammatory properties, are being studied as potential treatments. The purpose of this study was to shed light on the underlying molecular mechanisms and explore the medical application of NR and RV in diabetic wound healing. METHODS: 54 male Sprague-Dawley rats divided into control, diabetic (DM), Gel Base, DM-NR, DM-RV, and DM-NR + RV. Rats were orally administered 50 mg/kg/day of RV and 300 mg/kg/day of NR for 5 weeks. Following diabetes induction, their wounds were topically treated with 5 % NR and RV gel for 15 days. The wound closure rate, body weight, and serum lipid profiles were examined. Gene expression study evaluated UPR and pyroptosis-related genes (BIP, PERK, ATF6, IRE1alpha, sXBP1, CHOP, NLRP3, caspase-1, NFkappaB, and IL1-beta) in wound tissues, alongside histological assessment of cellular changes. RESULTS: NR and RV treatments greatly enhanced wound healing. Molecular investigation demonstrated UPR and pyroptosis marker modifications, suggesting UPR balance and anti-inflammatory effects. Histological investigation demonstrated decreased inflammation and increased re-epithelialization. The combination of NR and RV therapy had better results than either treatment alone. CONCLUSION: This study shows that NR and RV have therapeutic promise in treating diabetic wounds by addressing UPR dysregulation, and pyroptosis. The combination therapy is a viable strategy to improving the healing process, providing a multimodal intervention for diabetic skin ulcers. These findings pave the way for additional investigation and possible therapeutic applications, giving hope for better outcomes in diabetic wound care.
The Role and Mechanism of Nicotinamide Riboside in Oxidative Damage and a Fibrosis Model of Trabecular Meshwork Cells.[Pubmed:38546981]
Transl Vis Sci Technol. 2024 Mar 1;13(3):24.
PURPOSE: To investigate the potential effects and mechanism of Nicotinamide riboside (NR) on the oxidative stress and fibrosis model of human trabecular meshwork (HTM) cell line cells. METHODS: HTM cells were pretreated with NR, followed by the induction of oxidative injury and fibrosis by hydrogen peroxide (H2O2) and TGF-beta2, respectively. Cell viability was tested using Hoechst staining and MTT assays, cell proliferation was assessed by EdU assay, and cell apoptosis was detected by flow cytometry and western blotting. DCFH-DA and DHE probes were used to measure the level of reactive oxygen species (ROS), and MitoTracker staining was used to measure the mitochondrial membrane potential (MMP). Fibrotic responses, including cell migration and deposition of extracellular matrix (ECM) proteins, were detected via Transwell assays, qRT-PCR, and immunoblotting. RESULTS: NR pretreatment improved the viability, proliferation, and MMP of H2O2-treated HTM cells. Compared to cells treated solely with H2O2, HTM cells treated with both NR and H2O2, exhibited a reduced rate of apoptosis and generation of ROS. Compared with H2O2 pretreatment, NR pretreatment upregulated expression of the JAK2/Stat3 pathway but inhibited mitogen-activated protein kinase (MAPK) pathway expression. Moreover, 10-ng/mL TGF-beta2 promoted cell proliferation and migration, which were inhibited by NR pretreatment. Both qRT-PCR and immunoblotting showed that NR inhibited the expression of fibronectin in a TGF-beta2-induced fibrosis model. CONCLUSIONS: NR has a protective effect on oxidative stress and fibrosis in HTM cells, which may be related to the JAK2/Stat3 pathway and MAPK pathway. TRANSLATIONAL RELEVANCE: Our research provides the ongoing data for potential therapy of NAD+ precursors in glaucoma.
Nicotinamide ribose ameliorates myocardial ischemia/reperfusion injury by regulating autophagy and regulating oxidative stress.[Pubmed:38533432]
Exp Ther Med. 2024 Mar 7;27(5):187.
Nicotinamide riboside (NR) has been reported to play a protective role in myocardial ischemia-reperfusion (I/R) injury when used in association with other drugs; however, the individual effect of NR is unknown. In the present study Evan's blue/triphenyl tetrazolium chloride staining, hematoxylin and eosin staining, echocardiography, western blotting, reverse transcription-quantitative PCR, and the detection of myocardial injury-associated markers and oxidative stress metabolites were used to explore the ability of NR to alleviate cardiac I/R injury and the relevant mechanisms of action. In a mouse model of I/R injury, dietary supplementation with NR reduced the area of myocardial ischemic infarction, alleviated pathological myocardial changes, decreased inflammatory cell infiltration and attenuated the levels of mitochondrial reactive oxygen species (ROS) and creatine kinase myocardial band (CK-MB). In addition, echocardiography suggested that NR alleviated the functional damage of the myocardium caused by I/R injury. In H9c2 cells, NR pretreatment reduced the levels of lactate dehydrogenase, CK-MB, malondialdehyde, superoxide dismutase and ROS, and reduced cell mortality after the induction of hypoxia/reoxygenation (H/R) injury. In addition, the results indicated NR activated sirt 1 via the upregulation of nicotinamide adenine dinucleotide (NAD(+)) and protected the cells against autophagy. The sirt 1 inhibitor EX527 significantly attenuated the ability of NR to inhibit autophagy, but had no significant effect on the ROS content of the H9c2 cells. In summary, the present study suggests that NR protects against autophagy by increasing the NAD(+) content in the body via the sirt 1 pathway, although the sirt 1 pathway does not affect oxidative stress.
Insulin and glycolysis dependency of cardioprotection by nicotinamide riboside.[Pubmed:38528175]
Basic Res Cardiol. 2024 Mar 25.
Decreased nicotinamide adenine dinucleotide (NAD(+)) levels contribute to various pathologies such as ageing, diabetes, heart failure and ischemia-reperfusion injury (IRI). Nicotinamide riboside (NR) has emerged as a promising therapeutic NAD(+) precursor due to efficient NAD(+) elevation and was recently shown to be the only agent able to reduce cardiac IRI in models employing clinically relevant anesthesia. However, through which metabolic pathway(s) NR mediates IRI protection remains unknown. Furthermore, the influence of insulin, a known modulator of cardioprotective efficacy, on the protective effects of NR has not been investigated. Here, we used the isolated mouse heart allowing cardiac metabolic control to investigate: (1) whether NR can protect the isolated heart against IRI, (2) the metabolic pathways underlying NR-mediated protection, and (3) whether insulin abrogates NR protection. NR protection against cardiac IRI and effects on metabolic pathways employing metabolomics for determination of changes in metabolic intermediates, and (13)C-glucose fluxomics for determination of metabolic pathway activities (glycolysis, pentose phosphate pathway (PPP) and mitochondrial/tricarboxylic acid cycle (TCA cycle) activities), were examined in isolated C57BL/6N mouse hearts perfused with either (a) glucose + fatty acids (FA) ("mild glycolysis group"), (b) lactate + pyruvate + FA ("no glycolysis group"), or (c) glucose + FA + insulin ("high glycolysis group"). NR increased cardiac NAD(+) in all three metabolic groups. In glucose + FA perfused hearts, NR reduced IR injury, increased glycolytic intermediate phosphoenolpyruvate (PEP), TCA intermediate succinate and PPP intermediates ribose-5P (R5P) / sedoheptulose-7P (S7P), and was associated with activated glycolysis, without changes in TCA cycle or PPP activities. In the "no glycolysis" hearts, NR protection was lost, whereas NR still increased S7P. In the insulin hearts, glycolysis was largely accelerated, and NR protection abrogated. NR still increased PPP intermediates, with now high (13)C-labeling of S7P, but NR was unable to increase metabolic pathway activities, including glycolysis. Protection by NR against IRI is only present in hearts with low glycolysis, and is associated with activation of glycolysis. When activation of glycolysis was prevented, through either examining "no glycolysis" hearts or "high glycolysis" hearts, NR protection was abolished. The data suggest that NR's acute cardioprotective effects are mediated through glycolysis activation and are lost in the presence of insulin because of already elevated glycolysis.
Nicotinamide Riboside Augments Human Macrophage Migration via SIRT3-Mediated Prostaglandin E2 Signaling.[Pubmed:38474420]
Cells. 2024 Mar 5;13(5):455.
NAD(+) boosting via Nicotinamide riboside (NR) confers anti-inflammatory effects. However, its underlying mechanisms and therapeutic potential remain incompletely defined. Here, we showed that NR increased the expression of CC-chemokine receptor 7 (CCR7) in human M1 macrophages by flow cytometric analysis of cell surface receptors. Consequently, chemokine ligand 19 (CCL19, ligand for CCR7)-induced macrophage migration was enhanced following NR administration. Metabolomics analysis revealed that prostaglandin E2 (PGE2) was increased by NR in human monocytes and in human serum following in vivo NR supplementation. Furthermore, NR-mediated upregulation of macrophage migration through CCL19/CCR7 was dependent on PGE2 synthesis. We also demonstrated that NR upregulated PGE2 synthesis through SIRT3-dependent post-transcriptional regulation of cyclooxygenase 2 (COX-2). The NR/SIRT3/migration axis was further validated using the scratch-test model where NR and SIRT3 promoted more robust migration across a uniformly disrupted macrophage monolayer. Thus, NR-mediated metabolic regulation of macrophage migration and wound healing may have therapeutic potential for the topical management of chronic wound healing.
The mitochondrial UPR induced by ATF5 attenuates intervertebral disc degeneration via cooperating with mitophagy.[Pubmed:38472656]
Cell Biol Toxicol. 2024 Mar 13;40(1):16.
Intervertebral disc degeneration (IVDD) is an aging disease that results in a low quality of life and heavy socioeconomic burden. The mitochondrial unfolded protein response (UPR(mt)) take part in various aging-related diseases. Our research intents to explore the role and underlying mechanism of UPR(mt) in IVDD. Nucleus pulposus (NP) cells were exposed to IL-1beta and Nicotinamide riboside (NR) served as UPR(mt) inducer to treat NP cells. Detection of ATP, NAD + and NADH were used to determine the function of mitochondria. MRI, Safranin O-fast green and Immunohistochemical examination were used to determine the degree of IVDD in vivo. In this study, we discovered that UPR(mt) was increased markedly in the NP cells of human IVDD tissues than in healthy controls. In vitro, UPR(mt) and mitophagy levels were promoted in NP cells treated with IL-1beta. Upregulation of UPR(mt) by NR and Atf5 overexpression inhibited NP cell apoptosis and further improved mitophagy. Silencing of Pink1 reversed the protective effects of NR and inhibited mitophagy induced by the UPR(mt). In vivo, NR might attenuate the degree of IDD by activating the UPR(mt) in rats. In summary, the UPR(mt) was involved in IVDD by regulating Pink1-induced mitophagy. Mitophagy induced by the UPR(mt) might be a latent treated target for IVDD.
Integrated annotation prioritizes metabolites with bioactivity in inflammatory bowel disease.[Pubmed:38467837]
Mol Syst Biol. 2024 Apr;20(4):338-361.
Microbial biochemistry is central to the pathophysiology of inflammatory bowel diseases (IBD). Improved knowledge of microbial metabolites and their immunomodulatory roles is thus necessary for diagnosis and management. Here, we systematically analyzed the chemical, ecological, and epidemiological properties of ~82k metabolic features in 546 Integrative Human Microbiome Project (iHMP/HMP2) metabolomes, using a newly developed methodology for bioactive compound prioritization from microbial communities. This suggested >1000 metabolic features as potentially bioactive in IBD and associated ~43% of prevalent, unannotated features with at least one well-characterized metabolite, thereby providing initial information for further characterization of a significant portion of the fecal metabolome. Prioritized features included known IBD-linked chemical families such as bile acids and short-chain fatty acids, and less-explored bilirubin, polyamine, and vitamin derivatives, and other microbial products. One of these, Nicotinamide riboside, reduced colitis scores in DSS-treated mice. The method, MACARRoN, is generalizable with the potential to improve microbial community characterization and provide therapeutic candidates.
Nicotinamide riboside activates renal metabolism and protects the kidney in a model of Alport syndrome.[Pubmed:38464264]
bioRxiv [Preprint]. 2024 Feb 29:2024.02.26.580911.
Chronic kidney disease (CKD) is associated with renal metabolic disturbances, including impaired fatty acid oxidation (FAO). Nicotinamide adenine dinucleotide (NAD (+) ) is a small molecule that participates in hundreds of metabolism-related reactions. NAD (+) levels are decreased in CKD, and NAD (+) supplementation is protective. However, both the mechanism of how NAD (+) supplementation protects from CKD, as well as the cell types most responsible, are poorly understood. Using a mouse model of Alport syndrome, we show that Nicotinamide riboside (NR), an NAD (+) precursor, stimulates renal peroxisome proliferator-activated receptor alpha signaling and restores FAO in the proximal tubules, thereby protecting from CKD in both sexes. Bulk RNA-sequencing shows that renal metabolic pathways are impaired in Alport mice and dramatically activated by NR in both sexes. These transcriptional changes are confirmed by orthogonal imaging techniques and biochemical assays. Single nuclei RNA-sequencing and spatial transcriptomics, both the first of their kind from Alport mice, show that NAD (+) supplementation restores FAO in the proximal tubules with minimal effects on the podocytes. Finally, we also report, for the first time, sex differences at the transcriptional level in this Alport model. Male Alport mice had more severe inflammation and fibrosis than female mice at the transcriptional level. In summary, the data herein identify both the protective mechanism and location of NAD (+) supplementation in this model of CKD.
Characteristics of intestinal bacteriophages and their relationship with Bacteria and serum metabolites during quail sexual maturity transition.[Pubmed:38459523]
BMC Vet Res. 2024 Mar 8;20(1):93.
BACKGROUND: Bacteriophages are prokaryotic viruses that rank among the most abundant microbes in the gut but remain among the least understood, especially in quails. In this study, we surveyed the gut bacteriophage communities in 22 quails at different ages (days 20 and 70) using shotgun metagenomic sequencing. We then systematically evaluated the relationships with gut bacteria and host serum metabolites. RESULTS: We discovered that Myoviridae and Siphoviridae were the dominant bacteriophage families in quails. Through a random forest and LEfSe analysis, we identified 23 differential bacteriophages with overlapping presence. Of these, 21 bacteriophages (e.g., Enterococcus phage IME-EFm5 and Enterococcus phage IME-EFm1) showed higher abundances in the day 20 group, while two bacteriophages (Bacillus phage Silence and Bacillus virus WPh) were enriched in the day 70 group. These key bacteriophages can serve as biomarkers for quail sexual maturity. Additionally, the differential bacteriophages significantly correlated with specific bacterial species and shifts in the functional capacities of the gut microbiome. For example, Enterococcus phages (e.g., Enterococcus phage EFP01, Enterococcus phage IME-EFm5, and Enterococcus phage IME-EFm1) were significantly (P < 0.001, FDR) and positively correlated with Enterococcus faecalis. However, the relationships between the host serum metabolites and either bacteriophages or bacterial species varied. None of the bacteriophages significantly (P > 0.05, FDR) correlated with Nicotinamide riboside and triacetate lactone. In contrast, some differential bacterial species (e.g., Christensenella massiliensis and Bacteroides neonati) significantly (P < 0.05, FDR) correlated with Nicotinamide riboside and triacetate lactone. Furthermore, characteristic successional alterations in gut bacteriophages, bacteria, and host serum metabolites across different ages highlighted a sexual maturity transition coexpression network. CONCLUSION: This study improves our understanding of the gut bacteriophage characteristics in quails and offers profound insights into the interactions among gut bacteriophages, bacteria, and host serum metabolites during the quail's sexual maturity transition.


