3'-HydroxyflavanoneCAS# 92496-65-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 92496-65-6 | SDF | Download SDF |
| PubChem ID | 3534982.0 | Appearance | Powder |
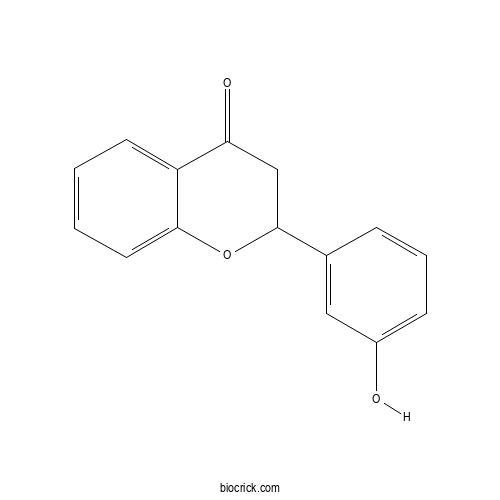
| Formula | C15H12O3 | M.Wt | 240.26 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3-hydroxyphenyl)-2,3-dihydrochromen-4-one | ||
| SMILES | C1C(OC2=CC=CC=C2C1=O)C3=CC(=CC=C3)O | ||
| Standard InChIKey | JVSPTYZZNUXJHN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O3/c16-11-5-3-4-10(8-11)15-9-13(17)12-6-1-2-7-14(12)18-15/h1-8,15-16H,9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3'-Hydroxyflavanone Dilution Calculator

3'-Hydroxyflavanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1622 mL | 20.8108 mL | 41.6216 mL | 83.2432 mL | 104.0539 mL |
| 5 mM | 0.8324 mL | 4.1622 mL | 8.3243 mL | 16.6486 mL | 20.8108 mL |
| 10 mM | 0.4162 mL | 2.0811 mL | 4.1622 mL | 8.3243 mL | 10.4054 mL |
| 50 mM | 0.0832 mL | 0.4162 mL | 0.8324 mL | 1.6649 mL | 2.0811 mL |
| 100 mM | 0.0416 mL | 0.2081 mL | 0.4162 mL | 0.8324 mL | 1.0405 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- D-mannuronic acid sodium salt
Catalog No.:BCX1439
CAS No.:921-56-2
- D-dimannuronic acid disodium salt
Catalog No.:BCX1438
CAS No.:34044-53-6
- D-trimannuronic acid trisodium salt
Catalog No.:BCX1437
CAS No.:66754-13-0
- D-tetramannuronic acid tetrasodium salt
Catalog No.:BCX1436
CAS No.:149511-34-2
- D-pentamannuronic acid pentasodium salt
Catalog No.:BCX1435
CAS No.:183668-50-0
- D-hexamannuronic acid hexasodium salt
Catalog No.:BCX1434
CAS No.:183668-52-2
- D-heptamannuronic acid heptasodium salt
Catalog No.:BCX1433
CAS No.:862694-97-1
- D-octamannuronic acid octasodium salt
Catalog No.:BCX1432
CAS No.:862694-98-2
- D-nonamannuronic acid nonasodium salt
Catalog No.:BCX1431
CAS No.:862694-99-3
- L-diguluronic acid disodium salt
Catalog No.:BCX1430
CAS No.:34044-54-7
- L-triguluronic acid trisodium salt
Catalog No.:BCX1429
CAS No.:66754-14-1
- L-tetraguluronic acid tetrasodium salt
Catalog No.:BCX1428
CAS No.:149511-37-5
- Tagitinin C
Catalog No.:BCX1441
CAS No.:59979-56-5
- Rubrofusarin triglucoside
Catalog No.:BCX1442
CAS No.:245724-07-6
- Vernoflexuoside
Catalog No.:BCX1443
CAS No.:57576-33-7
- Nicotinamide riboside
Catalog No.:BCX1444
CAS No.:1341-23-7
- 1β-Methoxydiversifolin
Catalog No.:BCX1445
CAS No.:110382-36-0
- 6-Carboxyl-7-hydroxy-2,3-dimethylchromone
Catalog No.:BCX1446
CAS No.:108170-57-6
- Neotame
Catalog No.:BCX1447
CAS No.:165450-17-9
- 4-Deoxyphorbol
Catalog No.:BCX1448
CAS No.:79083-67-3
- 16,17-Didehydroganoderic acid D
Catalog No.:BCX1449
CAS No.:1427189-02-3
- Stigmastanol
Catalog No.:BCX1450
CAS No.:83-45-4
- Methyl cis-11-eicosenoate
Catalog No.:BCX1451
CAS No.:2390-09-2
- 2'-Acetyltaxol
Catalog No.:BCX1452
CAS No.:92950-40-8
Regiospecificity of human UDP-glucuronosyltransferase isoforms in chalcone and flavanone glucuronidation determined by metal complexation and tandem mass spectrometry.[Pubmed:23713759]
J Nat Prod. 2013 Jun 28;76(6):1121-32.
The glucuronidation of a series of chalcones (2'-hydroxychalcone, 2',4'-dihydroxychalcone, 3,2'-dihydroxychalcone, 4,2'-dihydroxychalcone, and cardamonin) and their corresponding cyclized flavanones (7-hydroxyflavanone, 3'-hydroxyflavanone, 4'-hydroxyflavanone, and alpinetin) by eight human UDP-glucuronosyltransferase (UGT) 1A enzymes was evaluated. A postcolumn metal complexation LC-MS/MS strategy was used successfully to produce characteristic mass spectrometric product ions that were utilized in combination with elution order trends to identify chalcone and flavanone monoglucuronides unambiguously, thus allowing determination of the regioselectivities of the UGT1A isoforms. The presence of hydroxy groups on the A- or B-ring had a significant effect on the glucuronide product yield and the site where glucuronidation occurred. For example, for reaction with UGT1A9, formation of the 2'-O-glucuronide was increased for dihydroxychalcones with A-ring hydroxy substituents. In contrast, although UGT1A8 reacted with 3,2'-dihydroxychalcone and 4,2'-dihydroxychalcone to yield 2'-O-glucuronide products, the presence of a B-ring hydroxy group at the 4' position on cardamonin and 2',4'-dihydroxychalcone quenched the reaction at the OH-2' position. Moreover, the A-ring OH-4 group promoted glucuronidation at the 2' position for the reaction of 4,2'-dihydroxychalcone with UGT1A1 and 1A3. For UGT1A7, hydroxy group substituents on the chalcone A-ring also promoted cyclization and formation of the corresponding flavanone glucuronide.
Microbial metabolism. Part 12. Isolation, characterization and bioactivity evaluation of eighteen microbial metabolites of 4'-hydroxyflavanone.[Pubmed:21628902]
Chem Pharm Bull (Tokyo). 2011;59(6):692-7.
Fermentation of 4'-hydroxyflavanone (1) with fungal cultures, Beauveria bassiana (ATCC 13144 and ATCC 7159) yielded 6,3',4'-trihydroxyflavanone (2), 3',4'-dihydroxyflavanone 6-O-beta-D-4-methoxyglucopyranoside (3), 4'-hydroxyflavanone 3'-sulfate (4), 6,4'-dihydroxyflavanone 3'-sulfate (5) and 4'-hydroxyflavanone 6-O-beta-D-4-methoxyglucopyranoside (7). B. bassiana (ATCC 13144) and B. bassiana (ATCC 7159) in addition, gave one more metabolite each, namely, flavanone 4'-O-beta-D-4-methoxyglucopyranoside (6) and 6,4'-dihydroxyflavanone (8) respectively. Cunninghamella echinulata (ATCC 9244) transformed 1 to 6,4'-dihydroxyflavanone (8), flavanone-4'-O-beta-D-glucopyranoside (9), 3'-hydroxyflavanone 4'-sulfate (10), 3',4'-dihydroxyflavanone (11) and 4'-hydroxyflavanone-3'-O-beta-D-glucopyranoside (12). Mucor ramannianus (ATCC 9628) metabolized 1 to 2,4-trans-4'-hydroxyflavan-4-ol (13), 2,4-cis-4'-hydroxyflavan-4-ol (14), 2,4-trans-3',4'-dihydroxyflavan-4-ol (15), 2,4-cis-3',4'-dihydroxyflavan-4-ol (16), 2,4-trans-3'-hydroxy-4'-methoxyflavan-4-ol (17), flavanone 4'-O-alpha-D-6-deoxyallopyranoside (18) and 2,4-cis-4-hydroxyflavanone 4'-O-alpha-D-6-deoxyallopyranoside (19). Metabolites 13 and 14 were also produced by Ramichloridium anceps (ATCC 15672). The former was also produced by C. echinulata. Structures of the metabolic products were elucidated by means of spectroscopic data. None of the metabolites tested showed antibacterial, antifungal and antiprotozoal activities against selected organisms.
Quantitative morphometry of respiratory tract epithelial cells as a tool for testing chemopreventive agent efficacy.[Pubmed:20392991]
Anticancer Res. 2010 Mar;30(3):737-42.
Previously, we developed a 30-day transformation assay (focus inhibition, FIA) of rat tracheal epithelial (RTE) cells to identify cancer preventive agents. This study reports nuclear density (ND) as a morphometric biomarker for efficacy evaluation of at an early stage before transformed foci appear. Positive (oltipraz, D-carvone, fumaric acid, and 2-amino-4-methylpyridine or 2-A-MPR) and negative agents (myristoleic acid, anethole trithione, hydrocortisone, and 3'-hydroxyflavanone), identified from FIA, were tested for their effect on ND. RTE cells plated for 24 h were treated with a carcinogen, benzo[a] pyrene (B[a]P) or plus a test agent. The data based on the number of nuclei in agent-treated and control cells at day 14 showed that all FIA-positive agents inhibited ND from 23-66% at 0.3-1000.0 microM and except for myristoleic acid, all of the FIA-negative compounds were also negative in the morphometry assay. As there is strong correlation between the FIA and morphometry data, morphometry analysis is useful for rapid screening of potential chemopreventive agents.
Chiral separation of hydroxyflavanones in cyclodextrin-modified capillary zone electrophoresis using sulfated cyclodextrins as chiral selectors.[Pubmed:18342869]
J Chromatogr A. 2008 Apr 25;1188(2):301-7.
Chiral separations of three hydroxyflavanone aglycones, including 2'-, 3'-, and 4'-hydroxyflavanone, in capillary zone electrophoresis (CZE) using randomly sulfate-substituted beta-cyclodextrin (S-beta-CD) or dual cyclodextrin (CD) systems consisting of S-beta-CD and a neutral CD at low pH were investigated. The results indicate that S-beta-CD is an excellent chiral selector for enantioseparation of 2'-hydroxyflavanone and is a good chiral selector for 3'-hydroxyflavanone. Depending on the concentration of S-beta-CD ranging from 2.0 to 0.75% (w/v), the enantioresolution values were 10.5-19.5 and 1.8-3.4 for 2'- and 3'-hydroxyflavanone, respectively. The enantiomers of 4'-hydroxyflavanone could be effectively separated with S-beta-CD at a concentration of 2.0% (w/v) within 20 min. The enantioselectivity and enantioresolution follow the order 2'-hydroxyflavanone>>3'-hydroxyflavanone>4'-hydroxyflavanone. Alternatively, with the addition of sodium dodecyl sulfate (SDS) monomers at low concentrations in the electrophoretic system, enantioselectivity of these hydroxyflavanone aglycones could be enhanced with dual CD systems. In this case, SDS monomer acted as a complexing agent probably first with S-beta-CD and then subsequently with the analytes for increasing the effective electrophoretic mobility of the analytes towards the anode and as a selectivity controller for affecting the selectivity of hydroxyflavanones. Better enantioseparation between 2'-hydroxyflavanone and 3'-hydroxyflavanone could be achieved with a dual CD system consisting of S-beta-CD and gamma-CD than that with S-beta-CD and beta-CD. In addition, possible chiral recognition mechanisms of hydroxyflavanones are discussed.


