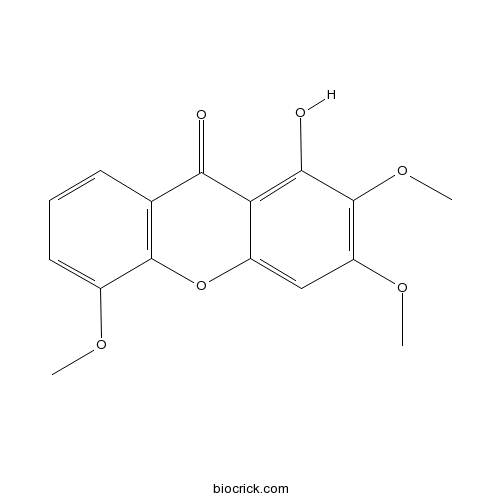
1-Hydroxy-2,3,5-trimethoxyxanthoneCAS# 22804-49-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22804-49-5 | SDF | Download SDF |
| PubChem ID | 5318372 | Appearance | Yellow powder |
| Formula | C16H14O6 | M.Wt | 302.3 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-hydroxy-2,3,5-trimethoxyxanthen-9-one | ||
| SMILES | COC1=CC=CC2=C1OC3=CC(=C(C(=C3C2=O)O)OC)OC | ||
| Standard InChIKey | FFVKXGZKJBHJMS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14O6/c1-19-9-6-4-5-8-13(17)12-10(22-15(8)9)7-11(20-2)16(21-3)14(12)18/h4-7,18H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 1-Hydroxy-2,3,5-trimethoxyxanthone (HM-1) has vasodilator action ,which involves both an endothelium-dependent mechanism involving NO and an endothelium-independent mechanism by inhibiting Ca(2+) influx through L-type voltage-operated Ca(2+) channels; a minor contribution to the effects of HM-1 may be related to inhibition of the protein kinase C-mediated release of intracellular Ca(2+) stores. 2. HM-1,at the concentration of 1 ug/mL, can effectively inhibit the osteoclast differentiation in a co-culture system with mouse osteoblastic calvarial cells and bone marrow cells, it exhibits significant inhibition of osteoclast differentiation even at a low concentration (0.01 ug/mL) in a dose-dependent manner, and it can significantly reduce the pit formation on the dentine slice compared with the control group. 3. HM-1 can protect mice from the acute lung injury induced by ipopolysaccharide (LPS), which is relative to the increasing of IκB-α protein expression and the suppressing of inducible nitric oxide synthase and cyclooxygenase-Ⅱ protein expression. |
| Targets | NO | 5-HT Receptor | Calcium Channel | COX | NOS | IkB | IKK |

1-Hydroxy-2,3,5-trimethoxyxanthone Dilution Calculator

1-Hydroxy-2,3,5-trimethoxyxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.308 mL | 16.5399 mL | 33.0797 mL | 66.1594 mL | 82.6993 mL |
| 5 mM | 0.6616 mL | 3.308 mL | 6.6159 mL | 13.2319 mL | 16.5399 mL |
| 10 mM | 0.3308 mL | 1.654 mL | 3.308 mL | 6.6159 mL | 8.2699 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3232 mL | 1.654 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ac-D-Trp-OH
Catalog No.:BCC3116
CAS No.:2280-01-5
- Ecdysterone 2,3:20,22-diacetonide
Catalog No.:BCN5074
CAS No.:22798-98-7
- Ecdysterone 20,22-monoacetonide
Catalog No.:BCN5073
CAS No.:22798-96-5
- EPI-001
Catalog No.:BCC6536
CAS No.:227947-06-0
- Z-D-Trp-OH
Catalog No.:BCC2748
CAS No.:2279-15-4
- Ethyl 3-(4-methoxyphenyl)propanoate
Catalog No.:BCN4038
CAS No.:22767-72-2
- (±)-HIP-B
Catalog No.:BCC7295
CAS No.:227619-65-0
- (±)-HIP-A
Catalog No.:BCC7294
CAS No.:227619-64-9
- Rubroside H
Catalog No.:BCN1857
CAS No.:227597-43-5
- Rubroside G
Catalog No.:BCN1856
CAS No.:227597-42-4
- Xanthobaccin A
Catalog No.:BCN1864
CAS No.:227596-81-8
- Mucrolidin
Catalog No.:BCN5072
CAS No.:227471-20-7
- 1,2,3,7-Tetramethoxyxanthone
Catalog No.:BCN7519
CAS No.:22804-52-0
- 3-(4-Hydroxy-3,5-dimethoxyphenyl)-1,2-propanediol
Catalog No.:BCN1480
CAS No.:22805-15-8
- Alisol K 23-acetate
Catalog No.:BCN3363
CAS No.:228095-18-9
- Boc-Met-OH.DCHA
Catalog No.:BCC2602
CAS No.:22823-50-3
- Hypoglaunine A
Catalog No.:BCN3086
CAS No.:228259-16-3
- Miconazole nitrate
Catalog No.:BCC9047
CAS No.:22832-87-7
- Boc-D-Val-OH
Catalog No.:BCC3466
CAS No.:22838-58-0
- Aspartame
Catalog No.:BCC8836
CAS No.:22839-47-0
- Pratensein
Catalog No.:BCN2918
CAS No.:2284-31-3
- 9-Epiblumenol B
Catalog No.:BCN5075
CAS No.:22841-42-5
- VULM 1457
Catalog No.:BCC7533
CAS No.:228544-65-8
- Ki8751
Catalog No.:BCC1116
CAS No.:228559-41-9
Mechanisms of the vasorelaxant effect of 1-hydroxy-2, 3, 5-trimethoxy-xanthone, isolated from a Tibetan herb, Halenia elliptica, on rat coronary artery.[Pubmed:17822718]
Life Sci. 2007 Sep 1;81(12):1016-23.
1-Hydroxy-2, 3, 5-trimethoxyxanthone (HM-1) is a xanthone isolated from Halenia elliptica, a Tibetan medicinal herb. HM-1 (0.33-42.1 microM) produced a concentration-dependent relaxation in rat coronary artery rings pre-contracted with 1 microM 5-hydroxytryptamine (5-HT), with an EC(50) of 1.67+/-0.27 microM. Removal of the endothelium significantly affected the vasodilator potency of HM-1, resulting in 46% decrease in E(max) value. The endothelium-dependent effects of HM-1 was confirmed when its vasorelaxant effect was inhibited after addition of nitric oxide synthase (NOS) inhibitor N(omega)-nitro-l-arginine methyl ester (100 microM) or the soluble guanylate cyclase inhibitor 1H-[1, 2, 4] oxadiazolo [4,3-alpha] quinoxalin-1-one (ODQ, 10 microM). Atropine (100 nM), flurbiprofen (10 microM), propranolol (100 microM), pyrilamine (10 microM), cimetidine (10 microM) and SQ22536 (100 microM) had no effect on the vasorelaxant activity of HM-1 indicated the non-involvement of other receptor/enzyme systems. In endothelium-denuded coronary artery rings, the vasorelaxant effect of HM-1 was unaffected by potassium channel blockers such as tetraethylammonium (10 mM), iberiotoxin (100 nM), barium chloride (100 microM) and 4-aminopyridine (1 mM). The involvement of Ca(2+) channel in 5-HT-primed artery ring preparations incubated with Ca(2+)-free buffer was confirmed when HM-1 (9.93 microM) partially abolished the CaCl(2)-induced vasoconstriction (87% inhibition in intact-endothelium artery rings; 50% inhibition in endothelium-denuded rings). In the KCl-primed preparations incubated with Ca(2+)-free buffer, HM-1 (9.93 microM) produced a 27.3% inhibition in endothelium-denuded rings. HM-1 (3.31-33.1 microM) had minimal relaxant effects (14.4%-20.3%) on the contractile response generated by 10 microM phorbol 12,13-diacetate (PDA) in Ca(2+)-free solutions, suggesting minimal effects on intracellular Ca(2+) mechanisms. These findings suggest the vasodilator action of HM-1 involved both an endothelium-dependent mechanism involving NO and an endothelium-independent mechanism by inhibiting Ca(2+) influx through L-type voltage-operated Ca(2+) channels; a minor contribution to the effects of HM-1 may be related to inhibition of the protein kinase C-mediated release of intracellular Ca(2+) stores.
Inhibitors of bone resorption from Halenia corniculata.[Pubmed:18704326]
Arch Pharm Res. 2008 Jul;31(7):850-5.
Eleven xanthones (1-11), three flavonoids (12-14) and three secoiridoids (15-17) were isolated from the aerial parts of Halenia corniculata. Among those compounds, 1-hydroxy-2,3,4,5-tetramethoxyxanthone (1), 1-hydroxy-2,3,4,7-tetramethoxyxanthone (2), 1-hydroxy-2,3,4,5,7-pentamethoxyxanthone (3), 1-Hydroxy-2,3,5-trimethoxyxanthone (4), 1,8-dihydroxy-3,5-dimethoxyxanthone (7), and luteolin (12), at the concentration of 1 microg/mL, effectively inhibited the osteoclast differentiation in a co-culture system with mouse osteoblastic calvarial cells and bone marrow cells. Notably, compounds 1, 3, and 4 exhibited, in a dose-dependent manner, significant inhibition of osteoclast differentiation even at a low concentration (0.01 microg/mL). All the inhibitory compounds, except for compound 7, significantly reduced the pit formation on the dentine slice compared with the control group. For the survival of the mature osteoclasts, compounds 1-4 and 12 (1 microg/mL), significantly decreased the survival number through induction of cell apoptosis, and compound 4 exhibited a significant inhibitory effect on osteoclast survival even at the low concentration of 0.1 microg/mL.


