EPI-001AR antagonist CAS# 227947-06-0 |
- Ro 31-8220
Catalog No.:BCC4295
CAS No.:125314-64-9
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
- Dequalinium Chloride
Catalog No.:BCC4998
CAS No.:522-51-0
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 227947-06-0 | SDF | Download SDF |
| PubChem ID | 4166922 | Appearance | Powder |
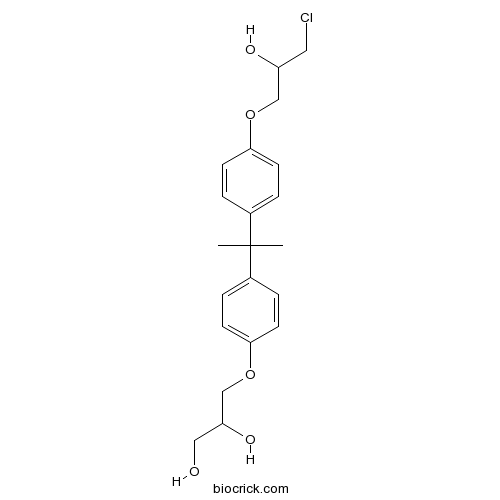
| Formula | C21H27ClO5 | M.Wt | 394.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | 3-[4-[2-[4-(3-chloro-2-hydroxypropoxy)phenyl]propan-2-yl]phenoxy]propane-1,2-diol | ||
| SMILES | CC(C)(C1=CC=C(C=C1)OCC(CO)O)C2=CC=C(C=C2)OCC(CCl)O | ||
| Standard InChIKey | HDTYUHNZRYZEEB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H27ClO5/c1-21(2,15-3-7-19(8-4-15)26-13-17(24)11-22)16-5-9-20(10-6-16)27-14-18(25)12-23/h3-10,17-18,23-25H,11-14H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

EPI-001 Dilution Calculator

EPI-001 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5324 mL | 12.6618 mL | 25.3235 mL | 50.647 mL | 63.3088 mL |
| 5 mM | 0.5065 mL | 2.5324 mL | 5.0647 mL | 10.1294 mL | 12.6618 mL |
| 10 mM | 0.2532 mL | 1.2662 mL | 2.5324 mL | 5.0647 mL | 6.3309 mL |
| 50 mM | 0.0506 mL | 0.2532 mL | 0.5065 mL | 1.0129 mL | 1.2662 mL |
| 100 mM | 0.0253 mL | 0.1266 mL | 0.2532 mL | 0.5065 mL | 0.6331 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
EPI-001 is a small-molecule inhibitor of androgen receptor N-terminal domain. EPI-001 could target the amino terminal domain of the AR and inhibit the protein-protein interactions which are necessary for AR transcriptional activity. The androgen receptor (AR) is involved in mediating the actions of male sex steroids. Amplification and over-expression of androgen receptor gene may result in hormone-refractory prostate cancer [1].
In vitro: EPI-001 inhibited the ligand-dependent ARGal4 transcriptional activity in LNCaP cells and the ligand-independent ARGal4 transcriptional activity in the CRPC C4-2 cell line. In a panel of cell lines, EPI-001 treatment decreased the mRNA expression level of AR protein after 8 and 16 h treatment, such as androgen sensitive PCa, CRPC, LNCap, C4-2, and LAPC4 cell line. EPI-001 dose-dependently inhibited the PCa/CRPC cell growth [2].
In vivo: Intravenous injection of EPI-001 significantly reduced the weight of benign prostates and blocked the growth of prostate cancer xenograft from noncastrated mature mice. EPI-001 caused tumor regression of CRPC. In male mice bearing LNCaP xenografts, after treated with EPI- 001 by i.v. injection for 2 weeks, the tumor size was less than half of the control group [3].
References:
Andersen R J, Mawji N R, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor[J]. Cancer cell, 2010, 17(6): 535-546.
Brand L J, Olson M E, Ravindranathan P, et al. EPI-001 is a selective peroxisome proliferator-activated receptor-gamma modulator with inhibitory effects on androgen receptor expression and activity in prostate cancer[J]. Oncotarget, 2015, 6(6): 3811.
Sadar M D. Small molecule inhibitors targeting the “achilles' heel” of androgen receptor activity[J]. Cancer research, 2011, 71(4): 1208-1213.
- Z-D-Trp-OH
Catalog No.:BCC2748
CAS No.:2279-15-4
- Ethyl 3-(4-methoxyphenyl)propanoate
Catalog No.:BCN4038
CAS No.:22767-72-2
- (±)-HIP-B
Catalog No.:BCC7295
CAS No.:227619-65-0
- (±)-HIP-A
Catalog No.:BCC7294
CAS No.:227619-64-9
- Rubroside H
Catalog No.:BCN1857
CAS No.:227597-43-5
- Rubroside G
Catalog No.:BCN1856
CAS No.:227597-42-4
- Xanthobaccin A
Catalog No.:BCN1864
CAS No.:227596-81-8
- Mucrolidin
Catalog No.:BCN5072
CAS No.:227471-20-7
- (3S,7S)-5,6-Dehydro-4''-de-O-methylcentrolobine
Catalog No.:BCN1481
CAS No.:227289-51-2
- AZ 11645373
Catalog No.:BCC7646
CAS No.:227088-94-0
- 9-Deacetyl-9-benzoyl-10-debenzoyl-4beta,20-epoxytaxchinin A
Catalog No.:BCN7676
CAS No.:227011-48-5
- Hyponine E
Catalog No.:BCC8999
CAS No.:226975-99-1
- Ecdysterone 20,22-monoacetonide
Catalog No.:BCN5073
CAS No.:22798-96-5
- Ecdysterone 2,3:20,22-diacetonide
Catalog No.:BCN5074
CAS No.:22798-98-7
- Ac-D-Trp-OH
Catalog No.:BCC3116
CAS No.:2280-01-5
- 1-Hydroxy-2,3,5-trimethoxyxanthone
Catalog No.:BCN6569
CAS No.:22804-49-5
- 1,2,3,7-Tetramethoxyxanthone
Catalog No.:BCN7519
CAS No.:22804-52-0
- 3-(4-Hydroxy-3,5-dimethoxyphenyl)-1,2-propanediol
Catalog No.:BCN1480
CAS No.:22805-15-8
- Alisol K 23-acetate
Catalog No.:BCN3363
CAS No.:228095-18-9
- Boc-Met-OH.DCHA
Catalog No.:BCC2602
CAS No.:22823-50-3
- Hypoglaunine A
Catalog No.:BCN3086
CAS No.:228259-16-3
- Miconazole nitrate
Catalog No.:BCC9047
CAS No.:22832-87-7
- Boc-D-Val-OH
Catalog No.:BCC3466
CAS No.:22838-58-0
- Aspartame
Catalog No.:BCC8836
CAS No.:22839-47-0
EPI-001 is a selective peroxisome proliferator-activated receptor-gamma modulator with inhibitory effects on androgen receptor expression and activity in prostate cancer.[Pubmed:25669987]
Oncotarget. 2015 Feb 28;6(6):3811-24.
The androgen receptor (AR) is a driver of prostate cancer (PCa) cell growth and disease progression. Therapies for advanced PCa exploit AR dependence by blocking the production or action of androgens, but these interventions inevitably fail via multiple mechanisms including mutation or deletion of the AR ligand binding domain (LBD). Thus, the development of new inhibitors which act through non-LBD interfaces is an unmet clinical need. EPI-001 is a bisphenol A-derived compound shown to bind covalently and inhibit the AR NH2-terminal domain (NTD). Here, we demonstrate that EPI-001 has general thiol alkylating activity, resulting in multilevel inhibitory effects on AR in PCa cell lines and tissues. At least one secondary mechanism of action associated with AR inhibition was found to be selective modulation of peroxisome proliferator activated receptor-gamma (PPARgamma). These multi-level effects of EPI-001 resulted in inhibition of transcriptional activation units (TAUs) 1 and 5 of the AR NTD, and reduced AR expression. EPI-001 inhibited growth of AR-positive and AR-negative PCa cell lines, with the highest sensitivity observed in LNCaP cells. Overall, this study provides new mechanistic insights to the chemical biology of EPI-001, and raises key issues regarding the use of covalent inhibitors of the intrinsically unstructured AR NTD.
Matrix-assisted laser desorption mass spectrometry imaging for the examination of imipramine absorption by Straticell-RHE-EPI/001 an artificial model of the human epidermis.[Pubmed:21480772]
Xenobiotica. 2011 Aug;41(8):735-42.
In order to assess the potential of matrix-assisted laser desorption mass spectrometry imaging for the examination of artificial skin models, the absorption of the tricyclic antidepressant imipramine into Straticell-RHE-EPI/001 an artificial model of the human epidermis has been studied. The presence of imipramine could be clearly discerned in treated samples by imaging the distribution of the protonated molecule at m/z 281.18 in samples taken 2 and 8 h after treatment. No clear evidence of biotransformation of imipramine in the artificial epidermal model was detected, although some signals that could potentially be assigned to the desmethyl metabolite were detected. Further work is required in order to investigate the reasons for the apparent low levels of metabolites detected.
Successful micronucleus testing with the EPI/001 3D reconstructed epidermis model: preliminary findings.[Pubmed:22266475]
Mutat Res. 2012 Mar 18;743(1-2):36-41.
Currently, the cosmetics industry relies on the results of in vitro genotoxicity tests to assess the safety of chemicals. Although the cytokinesis-block micronucleus (CBMN) test for the detection of cells that have divided once is routinely used and currently accepted by regulatory agencies, it has some limitations. Reconstituted human epidermis (RHE) is widely used in safety assessments because its physiological properties resemble those of the skin, and because it allows testing of substances such as hydrophobic compounds. Thus, the micronucleus test is being adapted for application in RHE-reconstructed tissues. Here we investigated whether two different reconstructed epidermis models (EPI/001 from Straticell, and RHE/S/17 from Skinethic) are suitable for application of the micronucleus test. We found that acetone does not modify micronucleus frequency, cell viability, and model structure, compared with non-treated RHE. Treatment of the EPI/001 model with mitomycin C and vinblastine resulted in a dose-dependent increase of micronucleus frequency as well as a decrease of tissue viability and of binucleated cell rate, while no changes of the epidermal structure were observed. The number of binucleated cells obtained with the RHE/S/17 model was too small to permit micronucleus testing. These results indicate that the proliferative rate of the tissue used is a critical parameter in performing the micronucleus test on a 3D model.
EPI-001, A Compound Active against Castration-Resistant Prostate Cancer, Targets Transactivation Unit 5 of the Androgen Receptor.[Pubmed:27356095]
ACS Chem Biol. 2016 Sep 16;11(9):2499-505.
Castration-resistant prostate cancer is the lethal condition suffered by prostate cancer patients that become refractory to androgen deprivation therapy. EPI-001 is a recently identified compound active against this condition that modulates the activity of the androgen receptor, a nuclear receptor that is essential for disease progression. The mechanism by which this compound exerts its inhibitory activity is however not yet fully understood. Here we show, by using high resolution solution nuclear magnetic resonance spectroscopy, that EPI-001 selectively interacts with a partially folded region of the transactivation domain of the androgen receptor, known as transactivation unit 5, that is key for the ability of prostate cells to proliferate in the absence of androgens, a distinctive feature of castration-resistant prostate cancer. Our results can contribute to the development of more potent and less toxic novel androgen receptor antagonists for treating this disease.


