(±)-HIP-BPotent, non-competitive EAAT inhibitor CAS# 227619-65-0 |
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- 17-AAG (KOS953)
Catalog No.:BCC2121
CAS No.:75747-14-7
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 227619-65-0 | SDF | Download SDF |
| PubChem ID | 15432002 | Appearance | Powder |
| Formula | C6H8N2O4 | M.Wt | 172.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in water | ||
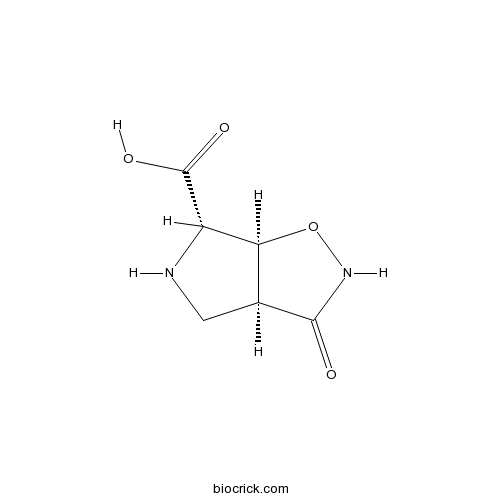
| Chemical Name | (3aS,6S,6aS)-3-oxo-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d][1,2]oxazole-6-carboxylic acid | ||
| SMILES | C1C2C(C(N1)C(=O)O)ONC2=O | ||
| Standard InChIKey | IOOKKDXVJPSSSC-HZLVTQRSSA-N | ||
| Standard InChI | InChI=1S/C6H8N2O4/c9-5-2-1-7-3(6(10)11)4(2)12-8-5/h2-4,7H,1H2,(H,8,9)(H,10,11)/t2-,3-,4-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, non-competitive excitatory amino acid transporter (EAAT) blocker. Preferentially inhibits glutamate-induced [3H]D-aspartate release (IC50 = 1.2 μM) rather than [3H]L-glutamate uptake (IC50 = 16.9 μM). Moderately selective; displays no affinity for NMDA and metabotropic glutamate receptors, and low affinity for AMPA and kainate receptors (IC50 values are 35 and 45 μM respectively). |

(±)-HIP-B Dilution Calculator

(±)-HIP-B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8092 mL | 29.0461 mL | 58.0923 mL | 116.1845 mL | 145.2306 mL |
| 5 mM | 1.1618 mL | 5.8092 mL | 11.6185 mL | 23.2369 mL | 29.0461 mL |
| 10 mM | 0.5809 mL | 2.9046 mL | 5.8092 mL | 11.6185 mL | 14.5231 mL |
| 50 mM | 0.1162 mL | 0.5809 mL | 1.1618 mL | 2.3237 mL | 2.9046 mL |
| 100 mM | 0.0581 mL | 0.2905 mL | 0.5809 mL | 1.1618 mL | 1.4523 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (±)-HIP-A
Catalog No.:BCC7294
CAS No.:227619-64-9
- Rubroside H
Catalog No.:BCN1857
CAS No.:227597-43-5
- Rubroside G
Catalog No.:BCN1856
CAS No.:227597-42-4
- Xanthobaccin A
Catalog No.:BCN1864
CAS No.:227596-81-8
- Mucrolidin
Catalog No.:BCN5072
CAS No.:227471-20-7
- (3S,7S)-5,6-Dehydro-4''-de-O-methylcentrolobine
Catalog No.:BCN1481
CAS No.:227289-51-2
- AZ 11645373
Catalog No.:BCC7646
CAS No.:227088-94-0
- 9-Deacetyl-9-benzoyl-10-debenzoyl-4beta,20-epoxytaxchinin A
Catalog No.:BCN7676
CAS No.:227011-48-5
- Hyponine E
Catalog No.:BCC8999
CAS No.:226975-99-1
- 3,6-Dimethoxyapigenin
Catalog No.:BCN4830
CAS No.:22697-65-0
- Emapunil
Catalog No.:BCC5521
CAS No.:226954-04-7
- LB42708
Catalog No.:BCC5344
CAS No.:226929-39-1
- Ethyl 3-(4-methoxyphenyl)propanoate
Catalog No.:BCN4038
CAS No.:22767-72-2
- Z-D-Trp-OH
Catalog No.:BCC2748
CAS No.:2279-15-4
- EPI-001
Catalog No.:BCC6536
CAS No.:227947-06-0
- Ecdysterone 20,22-monoacetonide
Catalog No.:BCN5073
CAS No.:22798-96-5
- Ecdysterone 2,3:20,22-diacetonide
Catalog No.:BCN5074
CAS No.:22798-98-7
- Ac-D-Trp-OH
Catalog No.:BCC3116
CAS No.:2280-01-5
- 1-Hydroxy-2,3,5-trimethoxyxanthone
Catalog No.:BCN6569
CAS No.:22804-49-5
- 1,2,3,7-Tetramethoxyxanthone
Catalog No.:BCN7519
CAS No.:22804-52-0
- 3-(4-Hydroxy-3,5-dimethoxyphenyl)-1,2-propanediol
Catalog No.:BCN1480
CAS No.:22805-15-8
- Alisol K 23-acetate
Catalog No.:BCN3363
CAS No.:228095-18-9
- Boc-Met-OH.DCHA
Catalog No.:BCC2602
CAS No.:22823-50-3
- Hypoglaunine A
Catalog No.:BCN3086
CAS No.:228259-16-3
Mechanism of inhibition of the glutamate transporter EAAC1 by the conformationally constrained glutamate analogue (+)-HIP-B.[Pubmed:22703277]
Biochemistry. 2012 Jul 10;51(27):5486-95.
Glutamate transporters play an important role in the regulation of extracellular glutamate concentrations in the mammalian brain and are, thus, promising targets for therapeutics. Despite this importance, the development of pharmacological tools has mainly focused on the synthesis of competitive inhibitors, which are amino acid analogues that bind to the substrate binding site. In this report, we describe the characterization of the mechanism of glutamate transporter inhibition by a constrained, cyclic glutamate analogue, (+)-3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d]isoxazole-6-carboxylic acid [(+)-(3aS,6S,6aS)-HIP-B]. Our results show that (+)-HIP-B is a nontransportable amino acid that inhibits glutamate transporter function in a mixed mechanism. Although (+)-HIP-B inhibits the glutamate-associated anion conductance, it has no effect on the leak anion conductance, in contrast to competitive inhibitors. Furthermore, (+)-HIP-B is unable to alleviate the effect of the competitive inhibitor dl-threo-beta-benzyloxyaspartic acid (TBOA), which binds to the substrate binding site. (+)-HIP-B is more potent in inhibiting forward transport compared to reverse transport. In a mutant transporter, which is activated by glutamine, but not glutamate, (+)-HIP-B still acts as an inhibitor, although this mutant transporter is insensitive to TBOA. Finally, we analyzed the effect of (+)-HIP-B on the pre-steady-state kinetics of the glutamate transporter. The results can be explained with a mixed mechanism at a site that may be distinct from the substrate binding site, with a preference for the inward-facing configuration of the transporter and slow inhibitor binding. (+)-HIP-B may represent a new paradigm of glutamate transporter inhibition that is based on targeting of a regulatory site.
Dissociation of [3H]L-glutamate uptake from L-glutamate-induced [3H]D-aspartate release by 3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d]isoxazole-4-carboxylic acid and 3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d]isoxazole-6-carboxylic acid, two conformationally constrained aspartate and glutamate analogs.[Pubmed:15322243]
Mol Pharmacol. 2004 Sep;66(3):522-9.
We characterized the interaction of two conformationally constrained aspartate and glutamate analogs, 3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d]isoxazole-4-carboxylic acid (HIP-A) and 3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-d]isoxazole-6-carboxylic acid (HIP-B), with excitatory amino acid transporters (EAATs) in rat brain cortex synaptosomes. HIP-A and HIP-B were potent and noncompetitive inhibitors of [(3)H]L-glutamate uptake, with IC(50) values (17-18 microM) very similar to that of the potent EAAT inhibitor dl-threo-beta-benzyloxyaspartic acid (TBOA). The two compounds had little effect in inducing [(3)H]D-aspartate release from superfused synaptosomes but they potently inhibited l-glutamate-induced [(3)H]D-aspartate release, thus behaving as EAAT blockers, not substrates, in a manner similar to those of TBOA and dihydrokainate (DHK). HIP-A and HIP-B, but not TBOA and DHK, unexpectedly inhibited L-glutamate-induced [(3)H]D-aspartate release with IC(50) values (1.2-1.6 microM) 10 times lower than those required to inhibit [(3)H]L-glutamate uptake. There is therefore a concentration window (1-3 microM) in which the two compounds significantly inhibited l-glutamate-induced release with very little effect on L-glutamate uptake. This selective inhibitory effect required quite long preincubation (>5 min) of synaptosomes with the drugs. At these low concentrations, however, HIP-A and HIP-B had no effect on the EAAT-mediated [(3)H]d-aspartate release induced by altering the ion gradients, indicating that they specifically affect some L-glutamate-triggered process(es)--different from L-glutamate translocation itself--responsible for the induction of reverse transport. These data are inconsistent with the classic model of facilitated exchange-diffusion and provide the first evidence that EAAT-mediated substrate uptake and substrate-induced EAAT-mediated reverse transport are independent. Compounds such as HIP-A and HIP-B could be useful to further clarify the mechanisms underlying these operating modes of transporters.
Synthesis and enantiopharmacology of new AMPA-kainate receptor agonists.[Pubmed:10514280]
J Med Chem. 1999 Oct 7;42(20):4099-107.
Regioisomeric 3-carboxyisoxazolinyl prolines [CIP-A (+/-)-6 and CIP-B (+/-)-7] and 3-hydroxyisoxazolinyl prolines [(+/-)-8 and (+/-)-9] were synthesized and assayed for glutamate receptor activity. The tests were carried out in vitro by means of receptor binding techniques, second messenger assays, and the rat cortical wedge preparation. CIP-A showed a good affinity for both 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionic acid (AMPA) and kainic acid (KAIN) receptors. These results were confirmed in the cortical slice model where CIP-A displayed an EC(50) value very close to that of AMPA. The convulsant properties of all the compounds were evaluated in vivo on DBA/2 mice after icv injection. CIP-A showed a convulsant activity, measured as tonus and clonus seizures, 18-65 times higher than that produced by AMPA. It was also quite active after ip administration, since it induced seizures in mice at doses as low as 3.2 nmol/mouse. On the basis of the above-reported results we prepared and tested the enantiomers of CIP-A and CIP-B, obtained by reacting (S)-3,4-didehydroproline and (R)-3,4-didehydroproline, respectively, with ethoxycarbonylformonitrile oxide. In all the tests the S-form, CIP-AS [(-)-6], emerged as the eutomer evidencing common stereochemical requirements with the reference compounds AMPA and KAIN. Through modeling studies, carried out on CIP-A, AMPA, and KAIN, active conformations for CIP-AS and AMPA at AMPA receptors as well as for CIP-AS and KAIN at KAIN receptors are suggested.


