1-Phenyl-2-propanolCAS# 14898-87-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

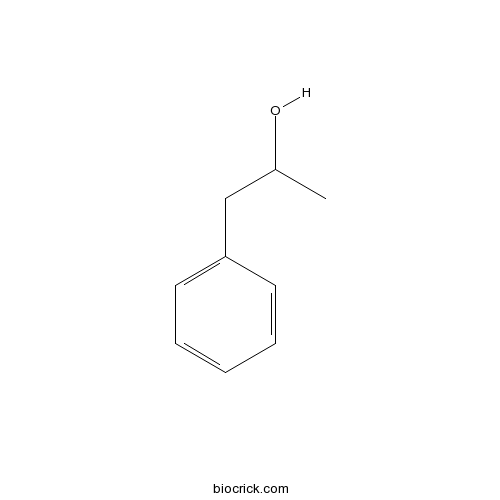
| Cas No. | 14898-87-4 | SDF | Download SDF |
| PubChem ID | 94185 | Appearance | Oil |
| Formula | C9H12O | M.Wt | 136.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-phenylpropan-2-ol | ||
| SMILES | CC(CC1=CC=CC=C1)O | ||
| Standard InChIKey | WYTRYIUQUDTGSX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H12O/c1-8(10)7-9-5-3-2-4-6-9/h2-6,8,10H,7H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | J Phys Chem A. 2006 Dec 14;110(49):13188-94.Rotational spectra and conformational structures of 1-phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone.[Pubmed: 17149832 ]
|

1-Phenyl-2-propanol Dilution Calculator

1-Phenyl-2-propanol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3421 mL | 36.7107 mL | 73.4214 mL | 146.8429 mL | 183.5536 mL |
| 5 mM | 1.4684 mL | 7.3421 mL | 14.6843 mL | 29.3686 mL | 36.7107 mL |
| 10 mM | 0.7342 mL | 3.6711 mL | 7.3421 mL | 14.6843 mL | 18.3554 mL |
| 50 mM | 0.1468 mL | 0.7342 mL | 1.4684 mL | 2.9369 mL | 3.6711 mL |
| 100 mM | 0.0734 mL | 0.3671 mL | 0.7342 mL | 1.4684 mL | 1.8355 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5-Hydroxy-1-(4-hydroxyphenyl)-7-phenyl-3-heptanone (AO 2210)
Catalog No.:BCN8786
CAS No.:105955-04-2
- Lanceolarin
Catalog No.:BCN8785
CAS No.:15914-68-8
- Specioside B
Catalog No.:BCN8784
CAS No.:126589-95-5
- 7-(4-hydroxy-3-methoxyphenyl)-1-phenylhept-4-en-3-one (DPHB)
Catalog No.:BCN8782
CAS No.:79559-60-7
- Dehydrojuncusol
Catalog No.:BCN8780
CAS No.:117824-04-1
- Isonardosinone
Catalog No.:BCN8779
CAS No.:27062-01-7
- Maculosidin
Catalog No.:BCN8778
CAS No.:522-19-0
- Silychristin B
Catalog No.:BCN8777
CAS No.:879325-58-3
- Yuanhunine
Catalog No.:BCN8776
CAS No.:104387-15-7
- 3-O-[5'''-O-feruloyl-beta-D-apiofuranosyl(1'''->2'')-beta-D-glucopyranosyl] rhamnocitrin
Catalog No.:BCN8775
CAS No.:148210-00-8
- Licoricesaponin H2(18beta,20alpha-Glycyrrhizic acid)
Catalog No.:BCN8774
CAS No.:118441-85-3
- Citrusin B
Catalog No.:BCN8773
CAS No.:105279-10-5
- Puerol B
Catalog No.:BCN8788
CAS No.:112343-17-6
- Gardenin D
Catalog No.:BCN8789
CAS No.:29202-00-4
- Emodin-8-O-beta-gentiobioside
Catalog No.:BCN8790
CAS No.:66466-22-6
- 5-Hydroxy-7-(4'-hydroxy-3'-methoxyphenyl)-1-phenyl-3-heptanone (DHPA)
Catalog No.:BCN8791
CAS No.:79559-61-8
- 4-Hydroxybenzoyl choline
Catalog No.:BCN8793
CAS No.:5094-31-5
- Patulitrin
Catalog No.:BCN8794
CAS No.:19833-25-1
- 7-(4-Hydroxyphenyl)-1-phenyl-4-hepten-3-one
Catalog No.:BCN8795
CAS No.:100667-52-5
- 3,7,25-Trihydroxycucurbita-5,23-dien-19-al
Catalog No.:BCN8799
CAS No.:85372-65-2
- Yuankanin
Catalog No.:BCN8755
CAS No.:77099-20-8
- New biochemical 1
Catalog No.:BCN8781
CAS No.:
- Complanatoside C
Catalog No.:BCN8783
CAS No.:
- New biochemical 2
Catalog No.:BCN8792
CAS No.:
Enhancing cofactor recycling in the bioconversion of racemic alcohols to chiral amines with alcohol dehydrogenase and amine dehydrogenase by coupling cells and cell-free system.[Pubmed:30536736]
Biotechnol Bioeng. 2019 Mar;116(3):536-542.
Alcohol dehydrogenase (ADH) and amine dehydrogenase (AmDH)-catalyzed one-pot cascade conversion of an alcohol to an amine provides a simple preparation of chiral amines. To enhance the cofactor recycling in this reaction, we report a new concept of coupling whole-cells with the cell-free system to enable separated intracellular and extracellular cofactor regeneration and recycling. This was demonstrated by the respective biotransformation of racemic 4-phenyl-2-butanol 1a and 1-Phenyl-2-propanol 1b to (R)-4-phenylbutan-2-amine 3a and (R)-1-phenylpropan-2-amine 3b. Escherichia coli cells expressing S-enantioselective CpsADH, R-enantioselective PfODH, and NADH oxidase (NOX) was developed to oxidize racemic alcohols 1a-b to ketones 2a-b with full conversion via intracellular NAD(+) recycling. AmDH and glucose dehydrogenase (GDH) were used to convert ketones 2a-b to amines (R)-3a-b with 89-94% conversion and 891-943 times recycling of NADH. Combining the cells and enzymes for the cascade transformation of racemic alcohols 1a-b gave 70% and 48% conversion to the amines (R)-3a and (R)-3b in 99% ee, with a total turnover number (TTN) of 350 and 240 for NADH recycling, respectively. Improved results were obtained by using the E. coli cells with immobilized AmDH and GDH: (R)-3a was produced in 99% ee with 71-84% conversion and a TTN of 1410-1260 for NADH recycling, the highest value so far for the ADH-AmDH-catalyzed cascade conversion of alcohols to amines. The concept might be generally applicable to this type of reactions.
Nano-amylose-2,3-bis(3,5-dimethylphenylcarbamate)-silica hybrid sol immobilized on open tubular capillary column for capillary electrochromatography enantioseparation.[Pubmed:29383728]
Electrophoresis. 2018 Apr;39(8):1086-1095.
The chiral organic-inorganic hybrid materials can exhibit a high loading, and the chiral selector nanoparticles can create efficient stationary phases for open-tubular capillary electrochromatography (OT-CEC). Hence, a novel protocol for the preparation of an OT column coated with nano-amylose-2,3-bis(3,5-dimethylphenylcarbamate) (nano-ABDMPC)-silica hybrid sol through in situ layer-by-layer self-assembly method was developed for CEC enantioseparation. By controlling the assembly cycle number of nano-ABDMPC-silica hybrid sol, a homogeneous, dense and stable coating was successfully prepared, which was confirmed by SEM and elemental analysis. As the main parameter influencing the chiral separating effect, the nano-ABDMPC bearing 3-(triethoxysilyl)propyl residues concentration was investigated. The experimental results showed that 10.0 mg/mL nano-ABDMPC bearing 3-(triethoxysilyl)propyl residues coated OT capillary column possessed chiral recognition ability toward the six enantiomers (phenylalanine, tyrosine, tryptophan, phenethyl alcohol, 1-Phenyl-2-propanol, and Troger's base) at some of the different conditions tested. Additionally, the coated OT column revealed adequate repeatability concerning run-to-run, day-to-day and column-to-column. These results demonstrated the promising applicability of nano-ABDMPC-silica hybrid sol coated OT column in CEC enantioseparations.
Conventional Chiralpak ID vs. capillary Chiralpak ID-3 amylose tris-(3-chlorophenylcarbamate)-based chiral stationary phase columns for the enantioselective HPLC separation of pharmaceutical racemates.[Pubmed:25271972]
Chirality. 2014 Nov;26(11):677-82.
A comparative enantioselective analysis using immobilized amylose tris-(3-chlorophenylcarbamate) as chiral stationary phase in conventional high-performance liquid chromatography (HPLC) with Chiralpak ID (4.6 mm ID x 250 mm, 5 microm silica gel) and micro-HPLC with Chiralpak ID-3 (0.30 mm ID x 150 mm, 3 microm silica gel) was conducted. Pharmaceutical racemates of 12 pharmacological classes, namely, alpha- and beta-blockers, anti-inflammatory drugs, antifungal drugs, dopamine antagonists, norepinephrine-dopamine reuptake inhibitors, catecholamines, sedative hypnotics, diuretics, antihistaminics, anticancer drugs, and antiarrhythmic drugs were screened under normal phase conditions. The effect of an organic modifier on the analyte retentions and enantiomer recognition was investigated. Baseline separation was achieved for 1-acenaphthenol, carprofen, celiprolol, cizolirtine carbinol, miconazole, tebuconazole, 4-hydroxy-3-methoxymandelic acid, 1-indanol, 1-(2-chlorophenyl)ethanol, 1-Phenyl-2-propanol, flavanone, 6-hydroxyflavanone, 4-bromogluthethimide, and pentobarbital on the 4.6 mm ID packed with a 5 microm silica column using conventional HPLC. Nonetheless, baseline separation was achieved for aminoglutethimide, naftopidil, and thalidomide on the 0.3 mm ID packed with a 3 microm silica capillary column.
Engineering of p450pyr hydroxylase for the highly regio- and enantioselective subterminal hydroxylation of alkanes.[Pubmed:24554642]
Angew Chem Int Ed Engl. 2014 Mar 17;53(12):3120-4.
Terminal-selective cytochrome P450pyr has been successfully engineered through directed evolution for the subterminal hydroxylation of alkanes with excellent regio- and enantioselectivity. A sensitive colorimetric high-throughput screening (HTS) assay was developed for the measurement of both the regioselectivity and the enantioselectivity of a hydroxylation reaction. By using the HTS assay and iterative saturation mutagenesis, sextuple-mutant P450pyrSM1 was created for the hydroxylation of n-octane (1) to give (S)-2-octanol (2) with 98 % ee and >99 % subterminal selectivity. The engineered P450 is the first enzyme for this type of highly selective alkane hydroxylation, being useful for the C-H activation and functionalization of alkanes and the preparation of enantiopure alcohols. Molecular modeling provided structure-based understanding of the fully altered regioselectivity and the excellent enantioselectivity. Another sextuple-mutant P450pyrSM2 catalyzed the hydroxylation of propylbenzene (3) to afford (S)-1-Phenyl-2-propanol (4) with 95 % ee and 98 % subterminal selectivity.
Chemical profiling of seized methamphetamine putatively synthesized from phenylacetic acid derivatives.[Pubmed:22989601]
Forensic Sci Int. 2013 Apr 10;227(1-3):42-4.
We report a case of seized crystalline methamphetamine (MA) samples showing unique drug profiles. The samples were mainly composed of (S)-(+)-MA, with each containing a slight amount of (R)-(-)-MA (enantiomeric excess: 99.2-99.4%). 1-Phenyl-2-propanol and N-methyl-2-phenylacetamide were detected as characteristic impurities. These analytical results suggested that the samples were synthesized as racemic MA by reductive amination of 1-phenyl-2-propanone, which was synthesized from phenylacetic acid, putatively prepared from phenylacetic acid ester, and then the racemic MA was optically resolved to the (+)-form-rich product. This proposed preparation route was in accordance with recent reports of seizures worldwide of the raw materials of MA such as phenylacetic acid derivatives, methylamine, and tartaric acid (optical resolving reagent).
Cross-examination of liquid-liquid extraction (LLE) and solid-phase microextraction (SPME) methods for impurity profiling of methamphetamine.[Pubmed:21376486]
Forensic Sci Int. 2012 Feb 10;215(1-3):175-8.
Impurities in 48 methamphetamine (MA) samples were analyzed by liquid-liquid extraction (LLE) and headspace solid-phase microextraction (HS-SPME) methods. MPS-2 autosampler was used to improve reproducibility of SPME method, and nonadecane (C(19)) diluted with potassium bromide (KBr) powder was used as an internal standard for standardizing retention time. Impurities identified by SPME method showed different patterns compared with LLE method. Non-volatile impurities like methamphetamine dimer were not identified by SPME method, but some volatile impurities like diphenylketone, caprolactam and lots of unknowns were identified only by SPME method. 1-Phenyl-2-propanone (P2P), 1-Phenyl-2-propanol and benzylcyanide peaks could be discriminated clearly by SPME method without interference of amphetamine, an artifact originates from MA degradation. Differences in the impurity patterns resulted in different clustering results. When 48 MA samples were classified into 5 LLE and 5 SPME clusters, cross-matching of the clusters resulted in 8 sub-clusters. It shows that combination of the different extraction methods can distinguish the differences which cannot be distinguished by LLE or SPME method alone, and can improve reliability of the profiling results.
Improved synthesis of (S)-1-phenyl-2-propanol in high concentration with coupled whole cells of Rhodococcus erythropolis and Bacillus subtilis on preparative scale.[Pubmed:20490950]
Appl Biochem Biotechnol. 2010 Nov;162(7):2075-86.
Bioreduction of 1-phenyl-2-propanone to prepare (S)-1-Phenyl-2-propanol, a useful pharmaceutical intermediate, was performed with growing cells of Rhodococcus erythropolis JX-021, giving 14 mM (1.9 g/L) product in 99% e.e. at 5 h in the catalysis of 15 mM substrate. The reduction stopped afterwards due to strong inhibition of substrate and formed product, a problem that is often encountered in biotransformation. While the substrate inhibition was solved by stepwise feeding, product inhibition was tackled by different methods: repeated removal of the product by centrifugation, by absorption with Amberlite XAD-7 resin, and by the use of dodecanol as the second phase gave the final product in 58, 68, and 61 mM in the catalysis of 80 mM substrate, respectively. The inhibition was caused by the partial permeabilization of cell membrane of R. erythropolis JX-021, and addition of NADPH or glucose 6-phosphate to such cell culture retained the reduction activity. Therefore, higher productivity in the reduction of 1 with resting cells of R. erythropolis JX-021 was achieved through cofactor regeneration and recycling by the addition of glucose and permeabilized cells of Bacillus subtilis BGSC 1A1 containing a glucose dehydrogenase, giving the product in 62 mM without addition of cofactor and 78 mM with the addition of 0.01 mM NADP(+) in the catalysis of 120 mM substrate. The product e.e. retained 99% during the process which showed industrial possibility.
Parameters influencing the release of tertiary alcohols from the surface of "spherical" dendrimers and "linear" stylomers by neighbouring-group-assisted hydrolysis of 2-carbamoylbenzoates.[Pubmed:19191231]
Chemistry. 2009;15(12):2846-60.
The influence of structural and physico-chemical parameters on the release of a volatile tertiary alcohol (2-methyl-1-Phenyl-2-propanol) by neighbouring-group-assisted cyclisation of 2-carbamoylbenzoates at neutral pH was investigated by comparing the covalent-bond cleavage from the surface of linear, comblike poly(propylene imine) "stylomers" and their corresponding spherical, globular dendrimers. Determination of the kinetic rate constants for the stepwise intramolecular cyclisation of the 2-carbamoylbenzoate moiety by using HPLC showed that the polarity of the conjugates, and thus their solubility in the aqueous reaction medium, has a stronger influence on the rates of hydrolysis than the size (generation) or shape (linear or spherical) of the macromolecules. Furthermore, structural modifications in close proximity to the release unit, such as the presence of functionalities with catalytic activity, have a strong impact on the release efficiency of the active molecules. An understanding of the physico-chemical parameters determining the local environment of the covalent-bond cleavage site is therefore an important prerequisite to transfer the characteristics of small molecules to larger structures such as oligomers and polymers and thus to design efficient macromolecular conjugates for the controlled delivery of bioactive compounds.
Purification and characterization of alcohol dehydrogenase reducing N-benzyl-3-pyrrolidinone from Geotrichum capitatum.[Pubmed:17368401]
J Biosci Bioeng. 2007 Feb;103(2):174-8.
(S)-N-Benzyl-3-pyrrolidinol is widely used in the synthesis of pharmaceuticals as a chiral building block. We produced 30 mM (S)-N-benzyl-3-pyrrolidinol (enantiometric excess > 99.9%) from the corresponding ketone N-benzyl-3-pyrrolidinone with more than 99.9% yield in 28 h of the resting-cell reaction of Geotrichum capitatum JCM 3908. NAD(+)-dependent alcohol dehydrogenase reducing N-benzyl-3-pyrrolidinone from G. capitatum JCM 3908 was purified to homogeneity by ammonium sulfate fractionation and a series of DEAE-Toyopearl, Butyl-Toyopearl, Superdex 200, and Hydroxyapatite column chromatographies. The results of SDS-PAGE and HPLC showed the enzyme to be a dimer with a molecular mass of 78 kDa. The purified enzyme produced (S)-N-benzyl-3-pyrrolidinol (e.e.>99.9%) from N-benzyl-3-pyrrolidinone. The enzyme reduced 2,3-butanedione, 2-hexanone, cyclohexanone, propionaldehyde, n-butylaldehyde, n-hexylaldehyde, n-octylaldehyde, n-valeraldehyde, and benzylacetone more effectively than it did N-benzyl-3-pyrrolidinone. No activity was detected towards N-benzyl-2-pyrrolidinone or 2-pyrrolidinone. The activity towards (R)-N-benzyl-3-pyrrolidinol was not detected under the assay conditions employed. The oxidizing activity of the enzyme was higher towards 2-propanol, 2-butanol, 2-pentanol, 2-hexanol, 3-hexanol, and 1-Phenyl-2-propanol than towards (S)-N-benzyl-3-pyrrolidinol. The K(m) values for N-benzyl-3-pyrrolidinone reduction and (S)-N-benzyl-3-pyrrolidinol oxidation were 0.13 and 8.47 mM, respectively. To our knowledge, this is the first time that an N-benzyl-3-pyrrolidinol/N-benzyl-3-pyrrolidinone oxidoreductase was purified from a eukaryote; moreover, this is the first report of (S)-N-benzyl-3-pyrrolidinol dehydrogenase activity in microorganisms. This enzyme showed features different from those of known prokaryotic N-benzyl-3-pyrrolidinone reductases. This enzyme will be very useful for the production of chiral compounds.
Rotational spectra and conformational structures of 1-phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone.[Pubmed:17149832]
J Phys Chem A. 2006 Dec 14;110(49):13188-94.
Microwave spectra have been recorded for 1-Phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone from 11 to 24 GHz using a Fourier-transform microwave spectrometer. Only one spectrum from a single conformational isomer was observed for each species. The rotational transitions in the spectrum of 1-phenyl-2-propanone were split into separate transitions arising from the A- and E-torsional levels of the methyl rotor. The fit of the E-state transitions to a "high-barrier" internal rotation Hamiltonian determines V3 = 238(1) cm-1 and rotor-axis angles of thetaa = 87.7(5) degrees, thetab = 50.0(5) degrees, and thetac = 40.0(5) degrees. Ab initio optimizations (MP2/6-31G**) and single-point calculations (MP2/6-311++G**) were used to model the structures of 1-Phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone. The lowest energy conformations of these species were found to be stabilized by weak OH-pi, NH-pi, and CH-pi hydrogen-bonding interactions. Moments of inertia, derived from the model structures, were used to assign the spectra to the lowest energy conformation of each species. A series of MP2/6-31G* partial optimizations along the internal rotation pathway were used to estimate the barrier to methyl rotation to be 355 cm-1 for 1-phenyl-2-propanone.
Enantioseparation of secondary alcohols by diastereoisomeric salt formation.[Pubmed:16385616]
Chirality. 2006 Feb;18(2):116-20.
A general method was found for the resolution of the racemic 1-phenyl-1-propanol (1) and 1-Phenyl-2-propanol (2) with various resolving agents. Monoesters of the alcohols were prepared, which were then reacted with different chiral bases. Successful optical resolutions were achieved only with the maleic acid monoesters (3 and 6). Alcohol 1 has been resolved to >99% enantiomeric excess by diastereoisomeric salt formation via its maleic acid monoester (3) using cinchonidine (9) as resolving agent. Alcohol 2 has been obtained in 98% enantiomeric excess by diastereoisomeric salt formation via its the maleic acid monoester (6) using (+)-dehydroabietylamine (11) as resolving agent.
Microbial degradation of illicit drugs, their precursors, and manufacturing by-products: implications for clandestine drug laboratory investigation and environmental assessment.[Pubmed:12842360]
Forensic Sci Int. 2003 Jun 24;134(1):62-71.
Chemicals associated with clandestine drug laboratories are often disposed of covertly into soil, sewerage systems, or public waste management facilities. There are two significant issues relating to such dumps of materials; they might contain valuable evidence as to drug manufacture, and they might be a source of pollution. This study presents initial findings in relation to the impact microorganisms from environmental sources have upon drugs, their precursors, and manufacturing by-products. The aim of this study was to identify which chemicals associated with clandestine drug laboratories persist in the environment in order to allow forensic drug chemists to link discarded residues with the method of manufacture, and to allow the environmental impact of clandestine drug laboratories to be assessed accurately. When exposed to soil microorganisms, phenyl-2-propanone (P2P) was rapidly metabolized into mixtures of 1-Phenyl-2-propanol, 1-phenyl-1,2-propanedione, 1-hydroxy-1-phenyl-2-propanone, 2-hydroxy-1-phenyl-1-propanone, and the two diastereoisomers of 1-phenyl-1,2-propanediol. On the other hand, when exposed under the same conditions, methylamphetamine sulphate (MAS) remained virtually unchanged. Implications relating to evidence gathering for forensic purposes and to environmental assessment of clandestine drug laboratories are discussed.


