DehydrojuncusolCAS# 117824-04-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117824-04-1 | SDF | Download SDF |
| PubChem ID | 5316773 | Appearance | Powder |
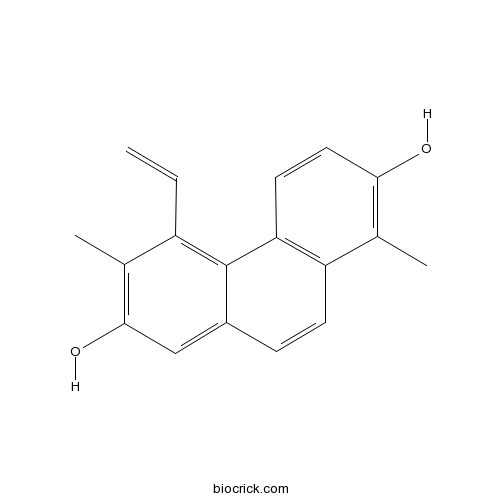
| Formula | C18H16O2 | M.Wt | 264.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-ethenyl-1,6-dimethylphenanthrene-2,7-diol | ||
| SMILES | CC1=C(C=CC2=C1C=CC3=CC(=C(C(=C32)C=C)C)O)O | ||
| Standard InChIKey | IZVFYHBVHNNKGE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O2/c1-4-13-10(2)17(20)9-12-5-6-14-11(3)16(19)8-7-15(14)18(12)13/h4-9,19-20H,1H2,2-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dehydrojuncusol, a new inhibitor of hepatitis C virus RNA replication, it inhibits infection of different HCV genotypes by targeting the NS5A protein and is active against resistant HCV variants frequently found in patients with treatment failure. |
| Targets | HCV |
| In vitro | Phenanthrenoids from Juncus acutus L., new natural lipopolysaccharide-inducible nitric oxide synthase inhibitors.[Pubmed: 17666857 ]Chem Pharm Bull (Tokyo). 2007 Aug;55(8):1264-6.
|
| In vivo | Anxiolytic effect of a novel 9,10-dihydrophenanthrene, juncuenin H, is associated with metabolic changes in cortical serotonin/dopamine levels in mice.[Pubmed: 30825572 ]Fitoterapia. 2019 Apr;134:165-171.Recent emergence of direct-acting antivirals (DAAs) targeting hepatitis C virus (HCV) proteins has considerably enhanced the success of antiviral therapy. However, the appearance of DAA-resistant-associated variants is a cause of treatment failure, and the high cost of DAAs renders the therapy not accessible in countries with inadequate medical infrastructures. Therefore, the search for new inhibitors with a lower cost of production should be pursued.
|

Dehydrojuncusol Dilution Calculator

Dehydrojuncusol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7836 mL | 18.9179 mL | 37.8358 mL | 75.6716 mL | 94.5895 mL |
| 5 mM | 0.7567 mL | 3.7836 mL | 7.5672 mL | 15.1343 mL | 18.9179 mL |
| 10 mM | 0.3784 mL | 1.8918 mL | 3.7836 mL | 7.5672 mL | 9.4589 mL |
| 50 mM | 0.0757 mL | 0.3784 mL | 0.7567 mL | 1.5134 mL | 1.8918 mL |
| 100 mM | 0.0378 mL | 0.1892 mL | 0.3784 mL | 0.7567 mL | 0.9459 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isonardosinone
Catalog No.:BCN8779
CAS No.:27062-01-7
- Maculosidin
Catalog No.:BCN8778
CAS No.:522-19-0
- Silychristin B
Catalog No.:BCN8777
CAS No.:879325-58-3
- Yuanhunine
Catalog No.:BCN8776
CAS No.:104387-15-7
- 3-O-[5'''-O-feruloyl-beta-D-apiofuranosyl(1'''->2'')-beta-D-glucopyranosyl] rhamnocitrin
Catalog No.:BCN8775
CAS No.:148210-00-8
- Licoricesaponin H2(18beta,20alpha-Glycyrrhizic acid)
Catalog No.:BCN8774
CAS No.:118441-85-3
- Citrusin B
Catalog No.:BCN8773
CAS No.:105279-10-5
- Genistein 7-O-beta-D-glucopyranoside-4'-O-[alpha-L-rhamnopyranosyl-(1->2)-beta-D-glucopyranoside]
Catalog No.:BCN8772
CAS No.:70404-42-1
- 1,7-Diphenyl-5-hydroxy-4,6-hepten-3-one
Catalog No.:BCN8771
CAS No.:87095-77-0
- Pueroside C
Catalog No.:BCN8770
CAS No.:112343-16-5
- Momordicoside X
Catalog No.:BCN8769
CAS No.:1333321-50-8
- Pueroside B
Catalog No.:BCN8768
CAS No.:100692-54-4
- 7-(4-hydroxy-3-methoxyphenyl)-1-phenylhept-4-en-3-one (DPHB)
Catalog No.:BCN8782
CAS No.:79559-60-7
- Specioside B
Catalog No.:BCN8784
CAS No.:126589-95-5
- Lanceolarin
Catalog No.:BCN8785
CAS No.:15914-68-8
- 5-Hydroxy-1-(4-hydroxyphenyl)-7-phenyl-3-heptanone (AO 2210)
Catalog No.:BCN8786
CAS No.:105955-04-2
- 1-Phenyl-2-propanol
Catalog No.:BCN8787
CAS No.:14898-87-4
- Puerol B
Catalog No.:BCN8788
CAS No.:112343-17-6
- Gardenin D
Catalog No.:BCN8789
CAS No.:29202-00-4
- Emodin-8-O-beta-gentiobioside
Catalog No.:BCN8790
CAS No.:66466-22-6
- 5-Hydroxy-7-(4'-hydroxy-3'-methoxyphenyl)-1-phenyl-3-heptanone (DHPA)
Catalog No.:BCN8791
CAS No.:79559-61-8
- 4-Hydroxybenzoyl choline
Catalog No.:BCN8793
CAS No.:5094-31-5
- Patulitrin
Catalog No.:BCN8794
CAS No.:19833-25-1
- 7-(4-Hydroxyphenyl)-1-phenyl-4-hepten-3-one
Catalog No.:BCN8795
CAS No.:100667-52-5
Dehydrojuncusol, a Natural Phenanthrene Compound Extracted from Juncus maritimus, Is a New Inhibitor of Hepatitis C Virus RNA Replication.[Pubmed:30842319]
J Virol. 2019 May 1;93(10). pii: JVI.02009-18.
Recent emergence of direct-acting antivirals (DAAs) targeting hepatitis C virus (HCV) proteins has considerably enhanced the success of antiviral therapy. However, the appearance of DAA-resistant-associated variants is a cause of treatment failure, and the high cost of DAAs renders the therapy not accessible in countries with inadequate medical infrastructures. Therefore, the search for new inhibitors with a lower cost of production should be pursued. In this context, the crude extract of Juncus maritimus Lam. was shown to exhibit high antiviral activity against HCV in cell culture. Bio-guided fractionation allowed the isolation and identification of the active compound, Dehydrojuncusol. A time-of-addition assay showed that Dehydrojuncusol significantly inhibited HCV infection when added after virus inoculation of HCV genotype 2a (50% effective concentration [EC50] = 1.35 microM). This antiviral activity was confirmed with an HCV subgenomic replicon, and no effect on HCV pseudoparticle entry was observed. Antiviral activity of Dehydrojuncusol was also demonstrated in primary human hepatocytes. No in vitro toxicity was observed at active concentrations. Dehydrojuncusol is also efficient on HCV genotype 3a and can be used in combination with sofosbuvir. Interestingly, Dehydrojuncusol was able to inhibit RNA replication of two frequent daclatasvir-resistant mutants (L31M or Y93H in NS5A). Finally, mutants resistant to Dehydrojuncusol were obtained and showed that the HCV NS5A protein is the target of the molecule. In conclusion, Dehydrojuncusol, a natural compound extracted from J. maritimus, inhibits infection of different HCV genotypes by targeting the NS5A protein and is active against resistant HCV variants frequently found in patients with treatment failure.IMPORTANCE Tens of millions of people are infected with hepatitis C virus (HCV) worldwide. Recently marketed direct-acting antivirals (DAAs) targeting HCV proteins have enhanced the efficacy of treatment. However, due to its high cost, this new therapy is not accessible to the vast majority of infected patients. Furthermore, treatment failures have also been reported due to the appearance of viral resistance. Here, we report on the identification of a new HCV inhibitor, Dehydrojuncusol, that targets HCV NS5A and is able to inhibit RNA replication of replicons harboring resistance mutations to anti-NS5A DAAs used in current therapy. Dehydrojuncusol is a natural compound isolated from Juncus maritimus, a halophilic plant species that is very common in coastlines worldwide. This molecule might serve as a lead for the development of a new therapy that is more accessible to hepatitis C patients in the future.
Anxiolytic effect of a novel 9,10-dihydrophenanthrene, juncuenin H, is associated with metabolic changes in cortical serotonin/dopamine levels in mice.[Pubmed:30825572]
Fitoterapia. 2019 Apr;134:165-171.
Two novel phenanthrenoids, juncuenin H (1) and dijuncuenin B (2), together with eight known phenanthrenoids, effusol (3), dehydroeffusol (4), juncusol (5), Dehydrojuncusol (6), juncuenin B (7), dehydrojuncuenin B (8), juncuenin A (9), and dehydrojuncuenin A (10), were isolated from the underground parts of Juncus setchuenensis. The structures of the compounds were determined by 1D and 2D NMR and mass spectroscopy. The anxiolytic activities of compounds 1, 6, 9, and 10 were evaluated. In order to explore the mechanisms underlying their anxiolytic activities, the levels of serotonin (5-HT), dopamine (DA), and their metabolites in the cerebral cortex and hippocampus of mice treated with compound 1 were determined by quantitative mass spectrometry. The mice treated with compound 1 had significantly lower levels of 5-HT, 3-methoxytyramine (3-MT), 5-hydroxyindole-3-acetic acid (5-HIAA), homovanillic acid (HVA), and 3, 4-dihydroxyphenylacetic acid (DOPAC) in the cerebral cortex than those of the vehicle control-treated mice. The levels of HVA and 5-HIAA in the hippocampus were also significantly lower in the mice treated with compound 1 than in the control group mice. These results suggest that the metabolic changes, reflected in the levels of DA and/or 5-HT, may contribute to the anxiolytic activity of the phenanthrenoids studied herein.
Phenanthrenes from Juncus inflexus with Antimicrobial Activity against Methicillin-Resistant Staphylococcus aureus.[Pubmed:27808510]
J Nat Prod. 2016 Nov 23;79(11):2814-2823.
The present study has focused on an investigation of the antibacterial effects of Juncus inflexus and the isolation and identification of its active compounds. Eleven phenanthrenes were isolated from a methanolic extract of the roots. Four compounds (jinflexins A-D, 1-4) are new natural products, while seven phenanthrenes [juncuenins A (5), B (6), and D (8), juncusol (7), dehydrojuncuenins A (9) and B (11), and Dehydrojuncusol (10)] were isolated for the first time from the plant. Jinflexin D (4) is a dimer with an unprecedented heptacyclic ring system. The absolute configurations of the new compounds were determined by TDDFT-ECD calculations, and their enantiomeric purity was checked by chiral HPLC analysis. Extracts of different polarity (n-hexane, dichloromethane, and ethyl acetate) were evaluated for their antimicrobial effects against methicillin-resistant Staphylococcus aureus, extended-spectrum beta-lactamase (ESBL)-producing Citrobacter freundii, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, multiresistant Acinetobacter baumannii, and Pseudomonas aeruginosa. The MIC values of the isolated compounds were determined by a microdilution method. Jinflexin B (2), juncusol (7), juncuenin D (8), and dehydrojuncuenin B (11) showed significant activity (MIC value range 12.5-100 mug/mL) against MRSA strains.
[Phenanthrenes from aerial part of Juncus setchuensis with anxiolyticactivity].[Pubmed:28875672]
Zhongguo Zhong Yao Za Zhi. 2016 Mar;41(6):1070-1074.
Ten phenanthrenes, two organic acids, one organic acid ester and one flavonoid were isolated from the aerial part of Juncus setchuensis by various chromatographic techniques usingsilica gel, polyamide, Sephadex LH-20 as solid phases, and preparative HPLC. Their structures were identified by MS and NMR spectroscopic data as effusol(1), juncusol(2), juncuenin D(3), dehydroeffusol(4), Dehydrojuncusol(5), juncuenin B(6),dehydrojuncuenin B(7), 2-methoxyl-7-hydroxyl-1-methyl-5-vinyl phenanthrene(8), 2-hydroxyl-7-carboxy-1-methyl-5-vinyl-9,10-dihydrophenanthrene(9), 2-hydroxyl-7-carboxyl-1-methyl-5-vinylphenanthrene(10), luteolin(11), vanillic acid(12), daphnetin(13), p-coumaric acid(14), respectively. Compound 13 was isolated from the genus Juncus for the first time and compounds 5, 8-12 were isolated from J. setchuensis for the first time. The elevated plus-maze(EPM) was used to evaluate the anxiolytic activity of compounds 6 and 7. Compound 6 at 5 mg*kg(-)(1) and 10 mg*kg(-)(1) showed anxiolytic activity as well as compound 7 at 10 mg*kg(-)(1) and 20 mg*kg(-)(1).
A novel antioxidant phenanthrenoid dimer from Juncus acutus L.[Pubmed:22360833]
Nat Prod Res. 2013;27(2):155-63.
A novel phenanthrenoid symmetrical dimer 8,8'-biDehydrojuncusol [1,1',6,6'-tetramethyl-5,5'-divinyl-8,8'-biphenanthrene-2,2',7,7'-tetraol], a related phenanthrenoid monomer, a phenolic chromone, and five flavonoids derivatives have been isolated from the halophyte Juncus acutus L., Juncaceae. The structure of the dimeric phenanthrenoid was determined on the basis of spectroscopic analyses, including 2D NMR spectroscopy, and by spectral correlations. The new dimer and the other isolated compounds bearing four phenolic hydroxy groups showed the significant in vitro antioxidant activity comparable with that of ascorbic acid using 2,2'-azino-bis[3-ethylbenzothiazoline-6-sulphonate] (ABTS) radical cation decolourisation assay. On the basis of the results from an in vitro anti-inflammatory assay using lipopolysaccharide-stimulated RAW264.7 macrophage cells linked with immunoblot analysis, it was found that dimerisation of Dehydrojuncusol [1,6-dimethyl-5-vinyl-8-phenanthrene-2,7-diol] molecule nearly nullified its inhibitory effect on the expression of the pro-inflammatory inducible nitric oxide synthase (iNOS) protein.
Phenanthrenes from Juncus effusus with anxiolytic and sedative activities.[Pubmed:22017742]
Nat Prod Res. 2012;26(13):1234-9.
Eight phenanthrenes, 7-carboxy-2-hydroxy-1-methyl-5-vinyl-phenanthrene (1); 2,7-dihydroxy-1-methyl-5-aldehyde-9,10-dihydrophenanthrene (2); dehydroeffusol (3); Dehydrojuncusol (4); 7-carboxy-2-hydroxy-1-methyl-5-vinyl-9,10-dihydrophenanthrene (5); 8-carboxy-2-hydroxy-1-methyl-5-vinyl-9,10-dihydrophenanthrene (6); effusol (7) and juncusol (8), were isolated from the aerial part of Juncus effusus. Compounds 1 and 2 were identified as new constituents. Compounds 7 and 8 showed anxiolytic and sedative activities.
Phenanthrenoids from Juncus acutus L., new natural lipopolysaccharide-inducible nitric oxide synthase inhibitors.[Pubmed:17666857]
Chem Pharm Bull (Tokyo). 2007 Aug;55(8):1264-6.
The novel natural product juncutol (1), 1,4,7-trimethyl-8,9-dihydro-4H-cyclopenta[def]phenanthrene-2,6-diol, along with the three related metabolites juncusol (2), Dehydrojuncusol (3), and 6-hydroxymethyl-1-methyl-5-vinyl-9,10-dihydrophenanthrene-2-ol (4), were isolated from the rhizomes of Juncus acutus L. (Juncaceae) growing in Egypt. The structural identity of 1 was determined on the basis of spectroscopic analyses, including 2D NMR spectroscopy. The inhibitory effect of these natural products on the expression of inducible nitric oxide synthase (iNOS) in lipopolysaccharide-stimulated RAW264.7 macrophage cells was determined for the first time. The unprecedented symmetrical compound juncutol (1) was found to be the most potent inhibitor against the induction of the proinflammatory iNOS protein.


