Gardenin DCAS# 29202-00-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29202-00-4 | SDF | Download SDF |
| PubChem ID | 3080750 | Appearance | Powder |
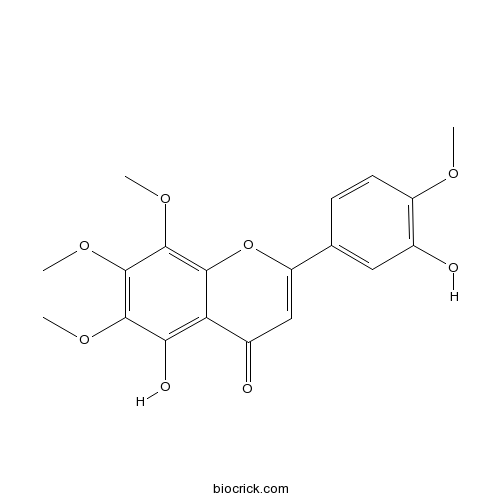
| Formula | C19H18O8 | M.Wt | 374.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-6,7,8-trimethoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=CC(=O)C3=C(C(=C(C(=C3O2)OC)OC)OC)O)O | ||
| Standard InChIKey | DQMSOZCDDAULPH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18O8/c1-23-12-6-5-9(7-10(12)20)13-8-11(21)14-15(22)17(24-2)19(26-4)18(25-3)16(14)27-13/h5-8,20,22H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gardenin D has antioxidant, and antiproliferative activities. |
| In vitro | Characterization of bioactive constituents from the gum resin of Gardenia lucida and its pharmacological potential.[Pubmed: 27899258 ]Biomed Pharmacother. 2017 Jan;85:444-456.
Inhibitory effects of phenolic compounds on CCl4-induced microsomal lipid peroxidation.[Pubmed: 2001725 ]Experientia. 1991 Feb 15;47(2):195-9.
|
| Structure Identification | Biochem Pharmacol. 1990 Aug 15;40(4):793-7.Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation.[Pubmed: 2386548 ]
|

Gardenin D Dilution Calculator

Gardenin D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6709 mL | 13.3547 mL | 26.7094 mL | 53.4188 mL | 66.7735 mL |
| 5 mM | 0.5342 mL | 2.6709 mL | 5.3419 mL | 10.6838 mL | 13.3547 mL |
| 10 mM | 0.2671 mL | 1.3355 mL | 2.6709 mL | 5.3419 mL | 6.6774 mL |
| 50 mM | 0.0534 mL | 0.2671 mL | 0.5342 mL | 1.0684 mL | 1.3355 mL |
| 100 mM | 0.0267 mL | 0.1335 mL | 0.2671 mL | 0.5342 mL | 0.6677 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Puerol B
Catalog No.:BCN8788
CAS No.:112343-17-6
- 1-Phenyl-2-propanol
Catalog No.:BCN8787
CAS No.:14898-87-4
- 5-Hydroxy-1-(4-hydroxyphenyl)-7-phenyl-3-heptanone (AO 2210)
Catalog No.:BCN8786
CAS No.:105955-04-2
- Lanceolarin
Catalog No.:BCN8785
CAS No.:15914-68-8
- Specioside B
Catalog No.:BCN8784
CAS No.:126589-95-5
- 7-(4-hydroxy-3-methoxyphenyl)-1-phenylhept-4-en-3-one (DPHB)
Catalog No.:BCN8782
CAS No.:79559-60-7
- Dehydrojuncusol
Catalog No.:BCN8780
CAS No.:117824-04-1
- Isonardosinone
Catalog No.:BCN8779
CAS No.:27062-01-7
- Maculosidin
Catalog No.:BCN8778
CAS No.:522-19-0
- Silychristin B
Catalog No.:BCN8777
CAS No.:879325-58-3
- Yuanhunine
Catalog No.:BCN8776
CAS No.:104387-15-7
- 3-O-[5'''-O-feruloyl-beta-D-apiofuranosyl(1'''->2'')-beta-D-glucopyranosyl] rhamnocitrin
Catalog No.:BCN8775
CAS No.:148210-00-8
- Emodin-8-O-beta-gentiobioside
Catalog No.:BCN8790
CAS No.:66466-22-6
- 5-Hydroxy-7-(4'-hydroxy-3'-methoxyphenyl)-1-phenyl-3-heptanone (DHPA)
Catalog No.:BCN8791
CAS No.:79559-61-8
- 4-Hydroxybenzoyl choline
Catalog No.:BCN8793
CAS No.:5094-31-5
- Patulitrin
Catalog No.:BCN8794
CAS No.:19833-25-1
- 7-(4-Hydroxyphenyl)-1-phenyl-4-hepten-3-one
Catalog No.:BCN8795
CAS No.:100667-52-5
- 3,7,25-Trihydroxycucurbita-5,23-dien-19-al
Catalog No.:BCN8799
CAS No.:85372-65-2
- Yuankanin
Catalog No.:BCN8755
CAS No.:77099-20-8
- New biochemical 1
Catalog No.:BCN8781
CAS No.:
- Complanatoside C
Catalog No.:BCN8783
CAS No.:
- New biochemical 2
Catalog No.:BCN8792
CAS No.:
- Polygalin J
Catalog No.:BCN8796
CAS No.:
- Pterodonoic acid
Catalog No.:BCN8797
CAS No.:62458-42-8
Characterization of bioactive constituents from the gum resin of Gardenia lucida and its pharmacological potential.[Pubmed:27899258]
Biomed Pharmacother. 2017 Jan;85:444-456.
In the present study we mined the information on Gardenia lucida (Dikamali) and identified seven polymethoxyflavones from its gum resin. We also investigated its antiproliferative and antioxidant potential. Xanthomicrol (8) found as potent DPPH scavenger (85.86+/-1.3%) along with strong ferric plummeting ability (53.60+/-2.0 FSE) and reducing potential (1.07+/-0.01) as compared to ascorbic acid. Gardenin B (5) strongly inhibit biochemical production of nitric oxide (IC50 10.59+/-0.4mug/mL) followed by 5-Desmethylnobiletin (7) and Gardenin E (10, IC5011.01+/-0.7-34.53+/-2.7mug/mL). Methanol extract, chloroform fraction and Acerosin (11), Gardenin D (9) and Gardenin B (5) exhibited superior antiproliferative activity against lung, breast, colon, hepatic and leukaemia cell lines as well as in keratinocytes (IC50 12.82+/-0.67-94.63+/-1.27mug/mL) whereas other fractions and isolated compounds moderately affect the cell proliferation (21.40+/-0.12-48.12+/-0.47%) with least and non-specific interaction against succinate dehydrogenase. Except compound 2, 3, 6, 8 and 11, others were found as a significant inhibitor of ODC (IC50 2.36+/-0.7-8.53+/-0.32mug/mL) with respect to DFMO (IC50 10.85+/-0.28mug/mL). In silico analysis also revealed enervated binding energy (-4.30 to -5.02kcal/mol) and inhibition constant (704.18-210.26muM) wherein 5, 7, 8, 9 and 10 showed specific interaction with the receptor while rest were non-specific. Except butanol fraction and Gardenin E, others were potently inhibited the cathepsin D activity with non-specific interaction and better binding energy (-5.78 to -7.24kcal/mol) and inhibition constant (57.87-4.90muM). In conclusion, it can be interpreted that isolated polymethoxyflavones (Gardenin B, 5-Desmethylnobiletin, Gardenin E) could be taken up as a lead for target specific studies. Methanol extract and chloroform fraction prevails in all the tested activity therefore cumulative and composite intervention of polymethoxyflavones present in it reveals its pharmacological attributes and traditional value.
Cytotoxic constituents of Isodon rubescens var. lushiensis.[Pubmed:14575445]
J Nat Prod. 2003 Oct;66(10):1391-4.
Five new ent-kaurane diterpenoids, ludongnins F-J (1-5), along with 10 known compounds, guidongnins A-C (6-8), angustifolin (9), 6-epiangustifolin (10), sculponeatin J (11), Gardenin D, 5,3',4'-trihydroxy-6,7,8-trimethoxyflavone, pedalitin, and quercetin, were isolated from the leaves of Isodon rubescens var. lushiensis. The structures of 1-5 were determined by spectroscopic analysis, as well as X-ray crystallographic analysis of 1. Compounds 1-11 were evaluated against K562 leukemia cells for their cytotoxic effects.
Inhibitory effects of phenolic compounds on CCl4-induced microsomal lipid peroxidation.[Pubmed:2001725]
Experientia. 1991 Feb 15;47(2):195-9.
The antiperoxidative effects of 35 phenolic compounds, most of them belonging to the flavonoid class, were investigated using CCl4-induced peroxidation of rat liver microsomes. This system was rather insensitive to gallic acid, methyl gallate and ellagic acid. Nevertheless it was inhibited by flavonoids and structure/activity relationships were established. The most potent compounds were Gardenin D, luteolin, apigenin (flavones), datiscetin, morin, galangin (flavonols), eriodictyol (flavanone), amentoflavone (biflavone) and the reference compound, (+)-catechin. The natural polymethoxyflavone Gardenin D has shown a potency comparable to that of (+)-catechin and higher than that of silybin. Thus, it may be considered as a new type of natural antioxidant with potential therapeutical applications.
Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation.[Pubmed:2386548]
Biochem Pharmacol. 1990 Aug 15;40(4):793-7.
Polymethoxylated flavones and C-glycosyl derivatives isolated from medicinal plants besides other flavonoid compounds were studied for their influence on lipid peroxidation induced by FeSO4+ cysteine in rat liver microsomes. A number of hydroxyflavones (e.g. luteolin); C-glycosyl-flavones (e.g. orientin); methoxyflavones (e.g. Gardenin D) and flavonols (e.g. datiscetin), as well as the flavanol leucocyanidol and the biflavone amentoflavone behaved as inhibitors of non-enzymic lipid peroxidation. Structure-activity relationships were established and it was observed that the structural features for active polyhydroxylated compounds were different from those of polymethoxylated flavones, antiperoxidative flavonoids possessing a high lipophilicity.


