PatulitrinCAS# 19833-25-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19833-25-1 | SDF | Download SDF |
| PubChem ID | 5483974 | Appearance | Powder |
| Formula | C22H22O13 | M.Wt | 494.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
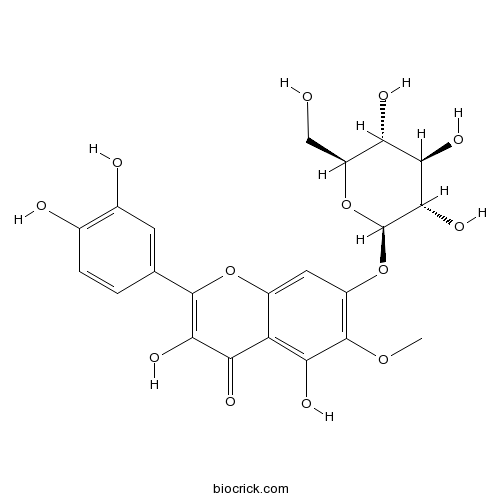
| Chemical Name | 2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-6-methoxy-7-[(2R,3S,4R,5R,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | ||
| SMILES | COC1=C(C=C2C(=C1O)C(=O)C(=C(O2)C3=CC(=C(C=C3)O)O)O)OC4C(C(C(C(O4)CO)O)O)O | ||
| Standard InChIKey | AFCDXKGLUDDXCK-NPSMOHFVSA-N | ||
| Standard InChI | InChI=1S/C22H22O13/c1-32-21-11(34-22-19(31)17(29)14(26)12(6-23)35-22)5-10-13(16(21)28)15(27)18(30)20(33-10)7-2-3-8(24)9(25)4-7/h2-5,12,14,17,19,22-26,28-31H,6H2,1H3/t12-,14-,17+,19-,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Patulitrin has antioxidant, anti-inflammatory, and larvicidal activities, oral and topical administration of patuletin and patulitrin inhibited acute inflammation in mice. |
| Targets | Antifection |
| In vitro | Larvicidal Activity against Aedes aegypti and Chemical Characterization of the Inflorescences of Tagetes patula.[Pubmed: 29362590 ]Evid Based Complement Alternat Med. 2017;2017:9602368.
Determination of antioxidant activity of extracts and fractions obtained from Galinsoga parviflora and Galinsoga quadriradiata, and a qualitative study of the most active fractions using TLC and HPLC methods.[Pubmed: 22085305 ]Nat Prod Res. 2012;26(17):1584-93.Taking into account the role of reactive oxygen species in the development of inflammation, and the application of the plants of genus Galinsoga Ruiz & Pav. in folk medicines for inflammatory states, we investigated and compared the antioxidant activities of particular Galinsoga extracts and fractions.
|
| In vivo | Effects of Flavonoids from French Marigold (Florets of Tagetes patula L.) on Acute Inflammation Model.[Pubmed: 24175111 ]Int J Inflam. 2013;2013:309493.
|
| Cell Research | Cytotoxic and antioxidant properties of phenolic compounds from Tagetes patula flower.[Pubmed: 25539472 ]Pharm Biol. 2015 May;53(5):672-81.
Tagetes patula Linn. (Asteraceae) (French Marigold) flowers are used by local practitioners for cancer treatment; however, it lacks scientific justification.
Identification of bioactive compounds in T. patula flower for cytotoxic and growth inhibition in human cancer cell lines along with its antioxidant properties using chemical and cell based systems.
|

Patulitrin Dilution Calculator

Patulitrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0227 mL | 10.1133 mL | 20.2265 mL | 40.4531 mL | 50.5663 mL |
| 5 mM | 0.4045 mL | 2.0227 mL | 4.0453 mL | 8.0906 mL | 10.1133 mL |
| 10 mM | 0.2023 mL | 1.0113 mL | 2.0227 mL | 4.0453 mL | 5.0566 mL |
| 50 mM | 0.0405 mL | 0.2023 mL | 0.4045 mL | 0.8091 mL | 1.0113 mL |
| 100 mM | 0.0202 mL | 0.1011 mL | 0.2023 mL | 0.4045 mL | 0.5057 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Hydroxybenzoyl choline
Catalog No.:BCN8793
CAS No.:5094-31-5
- 5-Hydroxy-7-(4'-hydroxy-3'-methoxyphenyl)-1-phenyl-3-heptanone (DHPA)
Catalog No.:BCN8791
CAS No.:79559-61-8
- Emodin-8-O-beta-gentiobioside
Catalog No.:BCN8790
CAS No.:66466-22-6
- Gardenin D
Catalog No.:BCN8789
CAS No.:29202-00-4
- Puerol B
Catalog No.:BCN8788
CAS No.:112343-17-6
- 1-Phenyl-2-propanol
Catalog No.:BCN8787
CAS No.:14898-87-4
- 5-Hydroxy-1-(4-hydroxyphenyl)-7-phenyl-3-heptanone (AO 2210)
Catalog No.:BCN8786
CAS No.:105955-04-2
- Lanceolarin
Catalog No.:BCN8785
CAS No.:15914-68-8
- Specioside B
Catalog No.:BCN8784
CAS No.:126589-95-5
- 7-(4-hydroxy-3-methoxyphenyl)-1-phenylhept-4-en-3-one (DPHB)
Catalog No.:BCN8782
CAS No.:79559-60-7
- Dehydrojuncusol
Catalog No.:BCN8780
CAS No.:117824-04-1
- Isonardosinone
Catalog No.:BCN8779
CAS No.:27062-01-7
- 7-(4-Hydroxyphenyl)-1-phenyl-4-hepten-3-one
Catalog No.:BCN8795
CAS No.:100667-52-5
- 3,7,25-Trihydroxycucurbita-5,23-dien-19-al
Catalog No.:BCN8799
CAS No.:85372-65-2
- Yuankanin
Catalog No.:BCN8755
CAS No.:77099-20-8
- New biochemical 1
Catalog No.:BCN8781
CAS No.:
- Complanatoside C
Catalog No.:BCN8783
CAS No.:
- New biochemical 2
Catalog No.:BCN8792
CAS No.:
- Polygalin J
Catalog No.:BCN8796
CAS No.:
- Pterodonoic acid
Catalog No.:BCN8797
CAS No.:62458-42-8
- 24-Hydroxymomordicine III
Catalog No.:BCN8798
CAS No.:
- Norwogonin 5,7,8-trimethyl ether
Catalog No.:BCN8800
CAS No.:23050-38-6
- Isoagarotetrol
Catalog No.:BCN8801
CAS No.:104060-61-9
- Sterebin A
Catalog No.:BCN8802
CAS No.:107647-14-3
Larvicidal Activity against Aedes aegypti and Chemical Characterization of the Inflorescences of Tagetes patula.[Pubmed:29362590]
Evid Based Complement Alternat Med. 2017;2017:9602368.
The crude acetone extract (CAE) of defatted inflorescences of Tagetes patula was partitioned into five semipurified fractions: n-hexane (HF), dichloromethane (DF), ethyl acetate (EAF), n-butanol (BF), and aqueous (AQF). BF was fractionated by reversed-phase polyamide column chromatography, obtaining 34 subfractions, which were subjected to HSCCC, where patuletin and Patulitrin were isolated. CAE and the fractions BF, EAF, DF, and AQF were analyzed by LC-DAD-MS, and patuletin and Patulitrin were determined as the major substances in EAF and BF, respectively. BF was also analyzed by HPLC and capillary electrophoresis (CE), and Patulitrin was again determined to be the main substance in this fraction. CAE and the semipurified fractions (750, 500, 300, 100, and 50 mg/L) were assayed for larvicidal activity against Aedes aegypti, with mortality rate expressed as percentage. All fractions except AQF showed insecticidal activity after 24 h exposure of larvae to the highest concentration. However, EAF showed the highest activity with more than 50% reduction in larval population at 50 mg/L. The insecticidal activity observed with EAF might have been due to the higher concentration of patuletin present in this fraction.
Simultaneous determination of thirteen flavonoids from Xiaobuxin-Tang extract using high-performance liquid chromatography coupled with electrospray ionization mass spectrometry.[Pubmed:26232587]
J Pharm Biomed Anal. 2015 Nov 10;115:214-24.
A simple and reliable high performance liquid chromatography coupled with electrospray ionization mass spectrometry (HPLC-ESI-MS) analysis method was established to simultaneously determine thirteen flavonoids of Xiaobuxing-Tang in intestine perfusate, namely onpordin, 3'-O-methylorobol, glycitein, patuletin, genistein, luteolin, quercetin, nepitrin, quercimeritrin, daidzin, Patulitrin, quercetagitrin and 3-glucosylisorhamnetin. Detection was performed on a quadrupole mass spectrometer equipped with an electrospray ionization (ESI) source operating in negative ionization mode. Negative ion ESI was used to form deprotonated molecules at m/z 315 for onpordin, m/z 299 for 3'-O-methylorobol, m/z 283 for glycitein, m/z 331 for patuletin, m/z 269 for genistein, m/z 285 for luteolin, m/z 301 for quercetin, m/z 477 for nepitrin, m/z 463 for quercimeritrin, m/z 461 for daidzin, m/z 493 for Patulitrin, m/z 479 for quercetagitrin, m/z 477 for 3-glucosylisorhamnetin and m/z 609.2 for rutin. The linearity, sensitivity, selectivity, repeatability, accuracy, precision, recovery and matrix effect of the assay were evaluated. The proposed method was successfully applied to simultaneous determination of these thirteen flavonoids, and using this method, the intestinal absorption profiles of thirteen flavonoids were preliminarily predicted.
Cytotoxic and antioxidant properties of phenolic compounds from Tagetes patula flower.[Pubmed:25539472]
Pharm Biol. 2015 May;53(5):672-81.
CONTEXT: Tagetes patula Linn. (Asteraceae) (French Marigold) flowers are used by local practitioners for cancer treatment; however, it lacks scientific justification. OBJECTIVE: Identification of bioactive compounds in T. patula flower for cytotoxic and growth inhibition in human cancer cell lines along with its antioxidant properties using chemical and cell based systems. MATERIALS AND METHODS: The T. patula flower methanol extract, its seven fractions, and three phenolic compounds including methyl protocatechuate (1), patuletin (2), and Patulitrin (3) were evaluated using sulforhodamine-B assay against HeLa, HT-144, NCI-H460, MCF-7, PC-3, and SF-268 human cancer cell lines. In parallel, antioxidant activity was evaluated using chemical (DPPH(.), deoxyribose, and lipid peroxidation assays) and cell-based chemiluminescence systems (human neutrophils and mice macrophages). RESULTS: The methanol extract and ethyl acetate insoluble fraction exhibited cytotoxic and growth inhibitory effects against HeLa in which 2 exhibited highest cell growth inhibition (GI50: 0.6 +/- 0.1 microg/ml) and cytotoxicity (LC50: 2.5 +/- 0.1 microg/ml). It also scavenged LOO(.) (IC50: 6.5 +/- 0.7 microg/ml) and [Formula: see text] (IC50: 27.5 +/- 1.3 mug/ml) in chemical systems and human neutrophils, respectively. However, 1 preferably scavenged H2O2-Cl(-) (IC50: 0.5 +/- 0.01 mug/ml) in mice macrophages. DISCUSSION AND CONCLUSION: Compound 2 from T. patula flower exhibited both growth inhibitory and cytotoxic properties while 1 and 3 were only growth inhibitory against HeLa. 1-3 also displayed antioxidant properties implying its probable role in growth inhibition/cytotoxic action. The present study provides scientific evidence for the use of T. patula flower in cancer treatment by traditional healer.
Effects of Flavonoids from French Marigold (Florets of Tagetes patula L.) on Acute Inflammation Model.[Pubmed:24175111]
Int J Inflam. 2013;2013:309493.
The major components patuletin and Patulitrin were isolated from French marigold (florets of Tagetes patula). Patuletin and Patulitrin were found to inhibit acute inflammation in mice. Oral administration of patuletin and Patulitrin significantly suppressed hind-paw edema induced by carrageenin and histamine, while topical application of patuletin and Patulitrin significantly inhibited ear edema induced by 12-O-tetradecanoylphorbol-13-acetate and arachidonic acid. Thus, oral and topical administration of patuletin and Patulitrin inhibited acute inflammation in mice. These results suggest the anti-inflammatory efficacy of French marigold.
First Report of Bacterial Soft Rot on Tagetes patula Caused by Dickeya dieffenbachiae in China.[Pubmed:30722334]
Plant Dis. 2013 Feb;97(2):282.
French marigold (Tagetes patula L.), originally from Mexico, is an annual herb widely planted in China because of its beautiful color, long flowering, and strong adaptability, and has been used widely for ornamentation and decorating. French marigold is also rich in patuletin, quercetagetin, and Patulitrin, and is therefore applied medicinally for treating colds and coughs. In early summer 2012, soft rot symptoms on French marigold were found at three flower nurseries in Guangzhou, Guangdong Province, P. R. China, and approximately 25% of the plants had the symptoms. The symptoms included tissue collapse of the stems at the soil line followed by wilting of the whole plants. Within 1 week, the infected stems showed vascular discoloration, turned brown and then inky black, and eventually the whole plant collapsed after the basal stem was infected. Bacteria were successfully isolated from eight symptomatic plants on nutrient agar media incubated at 30 degrees C for 48 h. Ten isolates were selected randomly for further characterization. They were gram negative, degraded pectate, negative for oxidase and positive for indole production, and utilized malonate, glucose, and sucrose but not glucopyranoside, trehalose, or palatinose. Polymerase chain reactions (PCR) were performed using the 16S primers 27f and 1495r (4) for molecular identification. Subsequent DNA sequencing showed that the representative tested strain TP1 (GenBank Accession No. JX575747) was 99% identical to that of Dickeya dieffenbachiae (JF419463) using BLASTn. Further genetic analysis of strain TP1 was performed targeting several housekeeping genes, i.e., dnaX (GenBank Accession No. JX575748) with primers dnaxf and dnaxr (3), gyrB (JX575749) with primers of gyrbf1 and gyrbr1 (1), and gapA (JX575750) with primers of gapa326f and gapa845r (2). They were most homologous to the sequences of D. dieffenbachiae, since they had 97%, 96%, and 97% identity with GenBank accessions GQ904794, JF311653, and GQ891968, respectively. Pathogenicity was confirmed by injecting all 10 original bacterial isolates into each of 10 French marigold seedlings, with approximately 100 mul of a bacterial suspension at 1 x 108 CFU/ml. Ten plants inoculated with 100 mul of sterile water served as controls. Plants were placed in a greenhouse at 30 to 32 degrees C and 90% relative humidity. Within 48 h, soft rot symptoms appeared on all inoculated seedlings, while the control plants appeared normal. D. dieffenbachiae was reisolated from the diseased tissues, and confirmed to be the same as the inoculated pathogen by conducting a 16S rDNA sequence comparison. Previously, black spot, botrytis blight, oedema, powdery mildew, southern bacterial wilt, and damping off have been found on T. patula. To our knowledge, it is the first report of a soft rot caused by D. dieffenbachiae on French marigold. Because of the popularity and high economic value of French marigold, identification of this progressing bacterial disease is important to maintain safe production and beautiful scenery. References: (1) B. R. Lin et al. Plant Dis. 96:452, 2012. (2) S. Nabhan et al. Plant Pathol. 61:498, 2012. (3) M. Slawiak et al. Eur. J. Plant Pathol. 125:245, 2009. (4) W. G. Weisburg. J. Bacteriol. 173:697, 1991.
Determination of antioxidant activity of extracts and fractions obtained from Galinsoga parviflora and Galinsoga quadriradiata, and a qualitative study of the most active fractions using TLC and HPLC methods.[Pubmed:22085305]
Nat Prod Res. 2012;26(17):1584-93.
Taking into account the role of reactive oxygen species in the development of inflammation, and the application of the plants of genus Galinsoga Ruiz & Pav. in folk medicines for inflammatory states, we investigated and compared the antioxidant activities of particular Galinsoga extracts and fractions. The compositions of the most active fractions were studied using thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC) methods. The extracts and fractions from Galinsoga parviflora Cav. and Galinsoga quadriradiata Ruiz et Pav. possess dose-dependent free radical-scavenging ability against DPPH* and superoxide radicals, as well as inhibitory effects on linoleic acid peroxidation in a manner comparable to gallic acid. In the most active fractions, flavonoids, Patulitrin, quercimeritrin, quercitagetrin and caffeoyl derivatives were detected. Our research demonstrates that the investigated herbs are an interesting source of preparations with significant antioxidant effects. Our results justify the use of both raw materials in inflammatory diseases, among others, due to their ability to prevent free radical-induced deleterious effects.
Bioassay-guided isolation of antioxidant agents with analgesic properties from flowers of Tagetes patula.[Pubmed:21284510]
Pharm Biol. 2011 May;49(5):516-25.
CONTEXT: Tagetes patula L. is one of the French marigold group of the Asteraceae family. It is recognized in folklore for its medicinal and pesticidal properties. OBJECTIVE: In search of more effective, but non-toxic compounds with antioxidative potential led to the bioassay guided isolation studies on the extracts of T. patula. MATERIALS AND METHODS: The bioassay on Tagetes patula flowers were carried out guided by in vitro antioxidant activity using DPPH assay. A minor but proven plant constituent methyl protocatechuate (1) was isolated by column chromatography, while patuletin (2) and Patulitrin (3) obtained in bulk by employing solvent partition of methanol extract. Derivatization of patuletin into benzoyl, cinnamoyl and methyl was conducted to establish the structure activity relationship (SAR). Analgesic activity of compound 2 was evaluated using acetic acid-induced writhing test and hot-plate test in mice. The toxicity of methanol extract and compound 2 were also determined. RESULTS: Polar extracts, fractions and phases demonstrated better antioxidant activity. The synthetic methyl protocatechuate (1) showed IC(50) value of 2.8 +/- 0.2 mug/mL, whereas patuletin (2) (IC(50) = 4.3 +/- 0.25 microg/mL) was comparable to quercetin and rutin but significantly better than Patulitrin (3) (IC(50) = 10.17 +/- 1.16 microg/mL). Toxicity test for the methanol extract and compound 2 did not elicit any behavioral changes or cause mortality in mice. Compound 2 also demonstrated mild analgesic property. DISCUSSION AND CONCLUSION: These findings demonstrate that the plant polar extracts and fractions possess significant antioxidant property with non-toxic effect. Compound 1 is a genuine plant constituent of T. patula.
Phenolic compounds with antioxidant activity from Anthemis tinctoria L. (Asteraceae).[Pubmed:17708435]
Z Naturforsch C J Biosci. 2007 May-Jun;62(5-6):326-30.
From the aerial parts of Anthemis tinctoria L. subsp. tinctoria var. pallida DC. (Asteraceae), one new cyclitol glucoside, conduritol F-1-O-(6'-O-E-p-caffeoyl)-beta-D-glucopyranoside (1), has been isolated together with four flavonoids, nicotiflorin (2), isoquercitrin (3), rutin (4) and Patulitrin (5). The structures of the isolated compounds were established by means of NMR, MS, and UV spectral analyses. Methanolic extract and pure isolated compounds were examined for their free radical, scavenging activity, using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free stable radical, and for their inhibitory activity toward soybean lipoxygenase, using linoleic acid as substrate. Compounds 1 and 5 showed a strong scavenging effect in the DPPH radical assay. In addition 5 also exhibited high inhibitory activity on soybean lipoxygenase.
Primary flavonoids in marigold dye: extraction, structure and involvement in the dyeing process.[Pubmed:17654539]
Phytochem Anal. 2008 Jan-Feb;19(1):46-51.
Flavonoids extracted from marigold flowers were investigated for their dyeing potential. Patulitrin (1) and patuletin (2) were isolated and their structures established using NMR and HPLC-MS. These compounds were identified as the main flavonoids present in the dyeing bath. Following the dyeing process, it was demonstrated that aglycone 2 bound more strongly to wool fibres than its glucoside 1. Moreover, analysis focused on 1 and 2 dynamics during plant growth revealed that these components were only found in flowers during and after flowering. The influence of growing location was also investigated and it appeared that cultivation under Mediterranean conditions enhanced biosynthesis of 1 and 2 . Finally, several solvents were tested for their potential to extract the flavonoids: the use of a water-ethanol mixture gave a high extraction efficiency and allowed selective extraction of 1 and 2. The implications of these results are discussed in relation to the development of marigold as a potential dyeing plant.
Acylated flavonol glycosides from the flower of Inula britannica.[Pubmed:10650074]
J Nat Prod. 2000 Jan;63(1):34-6.
Three new acylated flavonol glycosides, patuletin 7-O-(6' '-isobutyryl)glucoside (1), patuletin 7-O-[6' '-(2-methylbutyryl)]glucoside (2), and patuletin 7-O-(6' '-isovaleryl)glucoside (3), were isolated from the n-BuOH extract of Inula britannica flowers by bioassay-guided fractionation, together with other known flavonoids. The structures were elucidated by 1D and 2D NMR, FABMS, and other spectral analyses. The eight flavonoids, including new compounds (1-3), Patulitrin (7), nepitrin (8), axillarin (10), patuletin (11), and luteolin (12), showed profound antioxidant activity in DPPH assay and cytochrome-c reduction assay using HL-60 cell culture system.
Flavonols: pigments responsible for ultraviolet absorption in nectar guide of flower.[Pubmed:5050486]
Science. 1972 Aug 11;177(4048):528-30.
The petals of the black-eyed susan (Compositae: Rudbeckia hirta) contain three flavonol glucosides (6,7-dimethoxy-3',4',5-trihydroxyflavone-3-O-glucoside, Patulitrin, and quercetagetin). These compounds, which show intense spectral absorption at 340 to 380 nanometers, are restricted in distribution to the petal bases, which are ultraviolet absorbing as a result. Such ultraviolet-absorbing petal zones, known as "nectar guides," are invisible to us, but are visible and of orientation value to the pollinating insect that lands on the flower in search for food. This is the first time that ultraviolet absorption in a nectar guide has been interpreted in chemical terms. In view of the widespread occurrence of flavonols in flowers, it is suggested that these pigments serve specifically for demarcation of ultraviolet petal patterns visible and relevant to insects.


