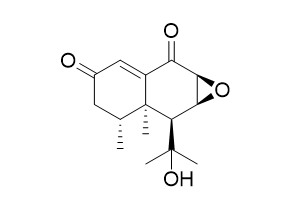
IsonardosinoneCAS# 27062-01-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 27062-01-7 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Oil |
| Formula | C15H20O4 | M.Wt | 264.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isonardosinone shows potential role in the treatment of neuroinflammation conditions, it inhibits NF-κB- and MAPK-mediated inflammatory pathways. |
| Targets | NF-κB | MAPK |
| In vitro | Nardosinone-Type Sesquiterpenes from the Hexane Fraction of Nardostachys jatamansi Attenuate NF-κB and MAPK Signaling Pathways in Lipopolysaccharide-Stimulated BV2 Microglial Cells.[Pubmed: 29616391 ]Inflammation. 2018 Aug;41(4):1215-1228.
|

Isonardosinone Dilution Calculator

Isonardosinone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7836 mL | 18.9179 mL | 37.8358 mL | 75.6716 mL | 94.5895 mL |
| 5 mM | 0.7567 mL | 3.7836 mL | 7.5672 mL | 15.1343 mL | 18.9179 mL |
| 10 mM | 0.3784 mL | 1.8918 mL | 3.7836 mL | 7.5672 mL | 9.4589 mL |
| 50 mM | 0.0757 mL | 0.3784 mL | 0.7567 mL | 1.5134 mL | 1.8918 mL |
| 100 mM | 0.0378 mL | 0.1892 mL | 0.3784 mL | 0.7567 mL | 0.9459 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Maculosidin
Catalog No.:BCN8778
CAS No.:522-19-0
- Silychristin B
Catalog No.:BCN8777
CAS No.:879325-58-3
- Yuanhunine
Catalog No.:BCN8776
CAS No.:104387-15-7
- 3-O-[5'''-O-feruloyl-beta-D-apiofuranosyl(1'''->2'')-beta-D-glucopyranosyl] rhamnocitrin
Catalog No.:BCN8775
CAS No.:148210-00-8
- Licoricesaponin H2(18beta,20alpha-Glycyrrhizic acid)
Catalog No.:BCN8774
CAS No.:118441-85-3
- Citrusin B
Catalog No.:BCN8773
CAS No.:105279-10-5
- Genistein 7-O-beta-D-glucopyranoside-4'-O-[alpha-L-rhamnopyranosyl-(1->2)-beta-D-glucopyranoside]
Catalog No.:BCN8772
CAS No.:70404-42-1
- 1,7-Diphenyl-5-hydroxy-4,6-hepten-3-one
Catalog No.:BCN8771
CAS No.:87095-77-0
- Pueroside C
Catalog No.:BCN8770
CAS No.:112343-16-5
- Momordicoside X
Catalog No.:BCN8769
CAS No.:1333321-50-8
- Pueroside B
Catalog No.:BCN8768
CAS No.:100692-54-4
- Formononetin-8-C-beta-D-apiofuranosyl-(1->6)-O-beta-D-glucopyranoside
Catalog No.:BCN8767
CAS No.:1147858-78-3
- Dehydrojuncusol
Catalog No.:BCN8780
CAS No.:117824-04-1
- 7-(4-hydroxy-3-methoxyphenyl)-1-phenylhept-4-en-3-one (DPHB)
Catalog No.:BCN8782
CAS No.:79559-60-7
- Specioside B
Catalog No.:BCN8784
CAS No.:126589-95-5
- Lanceolarin
Catalog No.:BCN8785
CAS No.:15914-68-8
- 5-Hydroxy-1-(4-hydroxyphenyl)-7-phenyl-3-heptanone (AO 2210)
Catalog No.:BCN8786
CAS No.:105955-04-2
- 1-Phenyl-2-propanol
Catalog No.:BCN8787
CAS No.:14898-87-4
- Puerol B
Catalog No.:BCN8788
CAS No.:112343-17-6
- Gardenin D
Catalog No.:BCN8789
CAS No.:29202-00-4
- Emodin-8-O-beta-gentiobioside
Catalog No.:BCN8790
CAS No.:66466-22-6
- 5-Hydroxy-7-(4'-hydroxy-3'-methoxyphenyl)-1-phenyl-3-heptanone (DHPA)
Catalog No.:BCN8791
CAS No.:79559-61-8
- 4-Hydroxybenzoyl choline
Catalog No.:BCN8793
CAS No.:5094-31-5
- Patulitrin
Catalog No.:BCN8794
CAS No.:19833-25-1
Nardosinone-Type Sesquiterpenes from the Hexane Fraction of Nardostachys jatamansi Attenuate NF-kappaB and MAPK Signaling Pathways in Lipopolysaccharide-Stimulated BV2 Microglial Cells.[Pubmed:29616391]
Inflammation. 2018 Aug;41(4):1215-1228.
Four nardosinone-type sesquiterpenes, nardosinone, Isonardosinone, kanshone E, and kanshone B, were isolated from the hexane fraction of Nardostachys jatamansi (Valerianaceae) methanol extract. The structures of these compounds were mainly established by analyzing the data obtained from nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). In this study, we investigated their anti-neuroinflammatory effects in lipopolysaccharide (LPS)-induced BV2 microglial cells. The results showed that nardosinone-type sesquiterpenes inhibited the production of pro-inflammatory mediators, such as nitric oxide (NO) and prostaglandin E2 (PGE2) in LPS-induced BV2 microglial cells. These inhibitory effects were correlated with the downregulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Moreover, these sesquiterpenes also attenuated the mRNA expression of pro-inflammatory cytokines including interleukin-1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha (TNF-alpha) in LPS-induced BV2 microglial cells. During the evaluation of the signaling pathways involved in these anti-neuroinflammatory effects, western blot analysis and DNA-binding activity assay revealed that the suppression of inflammatory reaction by these sesquiterpenes was mediated by the inactivation of nuclear factor-kappa B (NF-kappaB) pathway. These sesquiterpenes also suppressed the phosphorylation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) signaling pathways in LPS-stimulated BV2 microglial cells. Taken together, these four nardosinone-type sesquiterpenes inhibited NF-kappaB- and MAPK-mediated inflammatory pathways, demonstrating their potential role in the treatment of neuroinflammation conditions.
A Simple and Rapid UPLC-PDA Method for Quality Control of Nardostachys jatamansi.[Pubmed:29202512]
Planta Med. 2018 May;84(8):536-543.
Nardostachys jatamansi is a well-documented herbal agent used to treat digestive and neuropsychiatric disorders in oriental medicinal systems. However, few simple, rapid, and comprehensive methods were reported for quality assessment and control of N. jatamansi. Herein, a UPLC with photodiode array detection method was developed for both fingerprint investigation of N. jatamansi and simultaneous quantitative analysis of the six serotonin transporter modulatory constituents in N. jatamansi. For chromatographic fingerprinting, 24 common peaks were selected as characteristic peaks to assess the consistency of N. jatamansi samples from different retail sources. Six of the common peaks (5, 7, 12: , and 16: - 18: ) were identified as desoxo-narchinol A, buddleoside, Isonardosinone, nardosinone, kanshone H, and (-)-aristolone, respectively, by phytochemical investigation. Five of the six compounds significantly either enhanced or inhibited serotonin transporter activity, while (-)-aristolone (18: ) didn't show any serotonin transporter activity. In quantitative analysis, the six compounds showed good linearity (r > 0.999) within test ranges. The precision, expressed as relative standard deviation, was in the range of 0.25 - 2.77%, and the recovery of the method was in the range of 92 - 105%. The UPLC-photodiode array detection-based fingerprint analysis and quantitative methods reported here could be used for routine quality control of N. jatamansi.


