13-HydroxygermacroneCAS# 103994-29-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103994-29-2 | SDF | Download SDF |
| PubChem ID | 10399140 | Appearance | Cryst. |
| Formula | C15H22O2 | M.Wt | 234.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
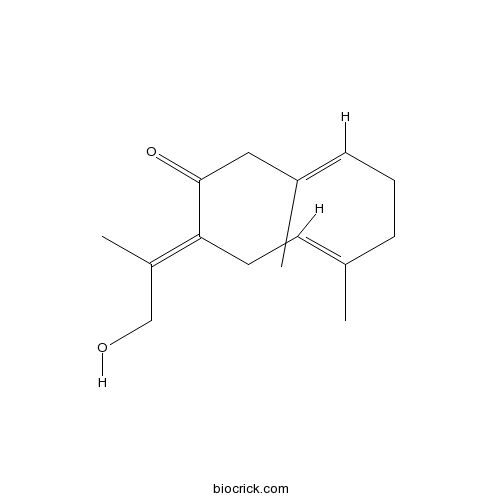
| Chemical Name | (3E,7E,10E)-10-(1-hydroxypropan-2-ylidene)-3,7-dimethylcyclodeca-3,7-dien-1-one | ||
| SMILES | CC1=CCC(=C(C)CO)C(=O)CC(=CCC1)C | ||
| Standard InChIKey | OYONKNQJEXRUQZ-UPXYLJBOSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 13-Hydroxygermacrone inhibits UVB-induced upregulation of the mRNA and protein expression levels of MMP-1, MMP-2, and MMP-3 in human keratinocytes (HaCaT). 2. 13-Hydroxygermacrone shows a protective effect against D-GalN/tumor necrosis factor-alpha-induced liver injury in mice at a dose of 50 mg/kg p.o. 3. 13-Hydroxygermacrone inhibits the increase in serum aspartate aminotransaminase and alanine aminotransaminase at a dose of 50 mg/kg p.o. |
| Targets | MMP(e.g.TIMP) | TNF-α |

13-Hydroxygermacrone Dilution Calculator

13-Hydroxygermacrone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.268 mL | 21.3402 mL | 42.6803 mL | 85.3606 mL | 106.7008 mL |
| 5 mM | 0.8536 mL | 4.268 mL | 8.5361 mL | 17.0721 mL | 21.3402 mL |
| 10 mM | 0.4268 mL | 2.134 mL | 4.268 mL | 8.5361 mL | 10.6701 mL |
| 50 mM | 0.0854 mL | 0.4268 mL | 0.8536 mL | 1.7072 mL | 2.134 mL |
| 100 mM | 0.0427 mL | 0.2134 mL | 0.4268 mL | 0.8536 mL | 1.067 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ceftiofur hydrochloride
Catalog No.:BCC8911
CAS No.:103980-44-5
- Esculentic acid
Catalog No.:BCN5856
CAS No.:103974-74-9
- 15-Nor-14-oxolabda-8(17),12-dien-18-oic acid
Catalog No.:BCN1637
CAS No.:1039673-32-9
- Oleanolic acid 3-O-beta-D-glucosyl-(1->3)-alpha-L-rhamnosyl(1->2)-alpha-L-arabinoside
Catalog No.:BCN8132
CAS No.:103956-33-8
- Lupeol caffeate
Catalog No.:BCN5855
CAS No.:103917-26-6
- Nodosin
Catalog No.:BCN5854
CAS No.:10391-09-0
- (-)-Isodocarpin
Catalog No.:BCN3280
CAS No.:10391-08-9
- Maxacalcitol
Catalog No.:BCC1730
CAS No.:103909-75-7
- 17β-Hydroxy-17-methylandrosta-4,9(11)-dien-3-one
Catalog No.:BCC8444
CAS No.:1039-17-4
- Mannioside A
Catalog No.:BCN5853
CAS No.:1038922-95-0
- MK-4827 Racemate
Catalog No.:BCC5179
CAS No.:1038915-75-1
- MK-4827 tosylate
Catalog No.:BCC4174
CAS No.:1038915-73-9
- 4'-Hydroxypiptocarphin A
Catalog No.:BCN7113
CAS No.:103994-39-4
- 4-Methoxyphenylacetic acid
Catalog No.:BCN8467
CAS No.:104-01-8
- Anethole
Catalog No.:BCN5373
CAS No.:104-46-1
- Cinnamyl alcohol
Catalog No.:BCN4967
CAS No.:104-54-1
- Cinnamaldehyde
Catalog No.:BCN6241
CAS No.:104-55-2
- Tussilagone
Catalog No.:BCN2770
CAS No.:104012-37-5
- 8alpha-Methacryloyloxybalchanin
Catalog No.:BCN4756
CAS No.:104021-39-8
- Trospium chloride
Catalog No.:BCC4582
CAS No.:10405-02-4
- LP533401 hcl
Catalog No.:BCC6377
CAS No.:1040526-12-2
- Quinovic acid 3-O-alpha-L-rhamnopyranoside
Catalog No.:BCN1636
CAS No.:104055-76-7
- Dihydroobovatin
Catalog No.:BCN3982
CAS No.:104055-79-0
- 3',5'-Diprenylgenistein
Catalog No.:BCN3572
CAS No.:104055-80-3
Germacrane sesquiterpenes isolated from the rhizome of Curcuma xanthorrhiza Roxb. inhibit UVB-induced upregulation of MMP-1, -2, and -3 expression in human keratinocytes.[Pubmed:25471012]
Arch Pharm Res. 2015 Oct;38(10):1752-60.
Four sesquiterpenes were isolated from the rhizome of Curcuma xanthorrhiza Roxb.: furanodiene (1), germacrone (2), furanodienone (3), and 13-Hydroxygermacrone (4). Importantly, this was the first time compounds 1 and 4 were isolated from this plant. The chemical structures of these compounds were determined using 1D- and 2D-nuclear magnetic resonance, infrared spectroscopy, and electron ionization mass spectrometry analyses. Among the isolated compounds, compounds 2 and 4 inhibited UVB-induced upregulation of the mRNA and protein expression levels of MMP-1, MMP-2, and MMP-3 in human keratinocytes (HaCaT). Moreover, this upregulation occurred in a dose-dependent manner over the range of 1-10 muM for each compound.
Medicinal foodstuffs. XXIX. Potent protective effects of sesquiterpenes and curcumin from Zedoariae Rhizoma on liver injury induced by D-galactosamine/lipopolysaccharide or tumor necrosis factor-alpha.[Pubmed:12033504]
Biol Pharm Bull. 2002 May;25(5):627-31.
The 80% aqueous acetone extract of Zedoariae Rhizoma was found to show a protective effect against D-galactosamine (D-GalN)/lipopolysaccharide-induced acute liver injury in mice. To clarify the active compounds, the principal constituents were examined and 11 sesquiterpenes (furanodiene, curdione, neocurdrione, dehydrocurdione, germacrone, 13-Hydroxygermacrone, curcumenol, isocurcumenol, aerugidiol, zedoarondiol, and curcumenone) and a diarylheptanoid (curcumin) were found to inhibit the increase in serum aspartate aminotransaminase and alanine aminotransaminase at a dose of 50 mg/kg p.o. in agreement with the previous in vitro studies, except for dehydrocurdione, aerugidiol, and zedoarondiol. In particular, curdione, neocurdione, curcumenol, and isocurcumenol potently inhibited the increase at a dose of 12.5 mg/kg p.o. Furthermore, the eight sesquiterpenes, furanodiene, curdione, neocurdione, dehydrocurdione, germacrone, 13-Hydroxygermacrone, curcumenol, and curcumenone, also showed a protective effect against D-GalN/tumor necrosis factor-alpha-induced liver injury in mice at a dose of 50 mg/kg p.o.
Anti-inflammatory sesquiterpenes from Curcuma zedoaria.[Pubmed:16901812]
Nat Prod Res. 2006 Jun;20(7):680-5.
From the methanolic extract of the rhizome of Curcuma zedoaria, we isolated anti-inflammatory sesquiterpene furanodiene (1) and furanodienone (2) along with new sesquiterpene compound 3 and known eight sesquiterpenes, zederone (4), curzerenone (5), curzeone (6), germacrone (7), 13-Hydroxygermacrone (8), dehydrocurdione (9), curcumenone (10), and zedoaronediol (11). Their structures were elucidated on the basis of spectroscopic data. The anti-inflammatory effect of isolated components on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation of mouse ears were examined. Compounds 1 and 2 suppressed the TPA-induced inflammation of mouse ears by 75% and 53%, respectively, at a dose of 1.0 micromol. Their activities are comparable to that of indomethacin, the normally used anti-inflammatory agent.


