TussilagoneCAS# 104012-37-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104012-37-5 | SDF | Download SDF |
| PubChem ID | 6438981 | Appearance | White powder |
| Formula | C23H34O5 | M.Wt | 390.51 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform and methan | ||
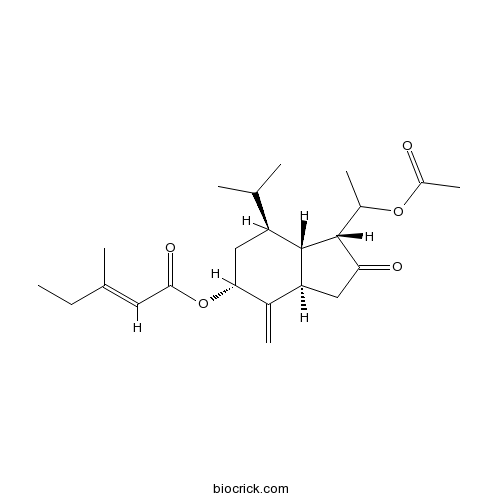
| Chemical Name | [(1R,3aR,5R,7S,7aS)-1-(1-acetyloxyethyl)-4-methylidene-2-oxo-7-propan-2-yl-3,3a,5,6,7,7a-hexahydro-1H-inden-5-yl] (E)-3-methylpent-2-enoate | ||
| SMILES | CCC(=CC(=O)OC1CC(C2C(C1=C)CC(=O)C2C(C)OC(=O)C)C(C)C)C | ||
| Standard InChIKey | CFUPNMDNSQIWBB-ACCKDKKQSA-N | ||
| Standard InChI | InChI=1S/C23H34O5/c1-8-13(4)9-21(26)28-20-11-17(12(2)3)23-18(14(20)5)10-19(25)22(23)15(6)27-16(7)24/h9,12,15,17-18,20,22-23H,5,8,10-11H2,1-4,6-7H3/b13-9+/t15?,17-,18-,20+,22+,23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tussilagone has anti-cancer, anti-oxidant and anti-inflammatory activities. Tussilagone inhibits dendritic cell function through the induction of heme oxygenase-1; it has potential treatment of neuro-inflammatory diseases through the inhibition of overproduction of nitric oxide and prostaglandin E(2). |
| Targets | NF-kB | HO-1 | TLR | IkB | Wnt/β-catenin | c-Myc | NO | NOS | COX | TNF-α | MAPK | p65 | IKK |
| In vitro | Tussilagone suppresses colon cancer cell proliferation by promoting the degradation of β-catenin.[Pubmed: 24269588]Biochem Biophys Res Commun. 2014 Jan 3;443(1):132-7.Abnormal activation of the Wnt/β-catenin signaling pathway frequently induces colon cancer progression. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 expression by tussilagone from Farfarae flos in BV-2 microglial cells.[Pubmed: 18481023]Arch Pharm Res. 2008 May;31(5):645-52.Activated microglia produce diverse neurotoxic factors such as nitric oxide (NO) and prostaglandin E(2) (PGE(2)) that may cause neurodegenerative diseases, including Alzheimer's disease and Parkinson's disease. |
| Kinase Assay | The anti-inflammatory effect of tussilagone, from Tussilago farfara, is mediated by the induction of heme oxygenase-1 in murine macrophages.[Pubmed: 19800419]Tussilagone inhibits dendritic cell functions via induction of heme oxygenase-1.[Pubmed: 25091622]Int Immunopharmacol. 2014 Oct;22(2):400-8.Sesquiterpenoid Tussilagone (TUS) has a variety of pharmacological activities, such as anti-oxidant, anti-cancer, and anti-inflammatory activities. Int Immunopharmacol. 2009 Dec;9(13-14):1578-84.Tussilagone (TSL), isolated from the flower of buds of Tussilago farfara (Compositae), is a sesquiterpenoid that is known to exert a variety of pharmacological activities. In the present study, we demonstrated that Tussilagone exerts anti-inflammatory activities in murine macrophages by inducing heme oxygenase-1 (HO-1) expression. Treatment of RAW264.7 cells with Tussilagone-induced HO-1 protein expression in a dose- and time-dependent manner without the induction of HO-1 mRNA expression. Tussilagone-mediated HO-1 protein induction was not inhibited by treatment with actinomycin D, a transcriptional inhibitor, but by cycloheximide, a translational inhibitor. Moreover, mitogen-activated protein kinases (MAPKs) inhibitors such as SB203580, SP600125, and U0126 did not block Tussilagone-mediated HO-1 protein expression, suggesting that the Tussilagone-mediated HO induction may be regulated at the translational level. Consistent with the notion that HO-1 has anti-inflammatory properties, Tussilagone inhibited the production of nitric oxide (NO), tumor necrosis factor (TNF)-alpha, and prostaglandin E2 (PGE2) as well as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression in lipopolysaccharide (LPS)-stimulated RAW264.7 cells and murine peritoneal macrophages. Inhibition of HO-1 activity by treatment with zinc protoporphyrin IX (ZnPP), a specific HO-1 inhibitor, abrogated the inhibitory effects of Tussilagone on the production of NO and PGE2 in LPS-stimulated RAW264.7 cells. Taken together, Tussilagone may be an effective HO-1 inducer that has anti-inflammatory effects, and a valuable compound for modulating inflammatory conditions. |

Tussilagone Dilution Calculator

Tussilagone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5608 mL | 12.8038 mL | 25.6075 mL | 51.2151 mL | 64.0188 mL |
| 5 mM | 0.5122 mL | 2.5608 mL | 5.1215 mL | 10.243 mL | 12.8038 mL |
| 10 mM | 0.2561 mL | 1.2804 mL | 2.5608 mL | 5.1215 mL | 6.4019 mL |
| 50 mM | 0.0512 mL | 0.2561 mL | 0.5122 mL | 1.0243 mL | 1.2804 mL |
| 100 mM | 0.0256 mL | 0.128 mL | 0.2561 mL | 0.5122 mL | 0.6402 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cinnamaldehyde
Catalog No.:BCN6241
CAS No.:104-55-2
- Cinnamyl alcohol
Catalog No.:BCN4967
CAS No.:104-54-1
- Anethole
Catalog No.:BCN5373
CAS No.:104-46-1
- 4-Methoxyphenylacetic acid
Catalog No.:BCN8467
CAS No.:104-01-8
- 4'-Hydroxypiptocarphin A
Catalog No.:BCN7113
CAS No.:103994-39-4
- 13-Hydroxygermacrone
Catalog No.:BCN3556
CAS No.:103994-29-2
- Ceftiofur hydrochloride
Catalog No.:BCC8911
CAS No.:103980-44-5
- Esculentic acid
Catalog No.:BCN5856
CAS No.:103974-74-9
- 15-Nor-14-oxolabda-8(17),12-dien-18-oic acid
Catalog No.:BCN1637
CAS No.:1039673-32-9
- Oleanolic acid 3-O-beta-D-glucosyl-(1->3)-alpha-L-rhamnosyl(1->2)-alpha-L-arabinoside
Catalog No.:BCN8132
CAS No.:103956-33-8
- Lupeol caffeate
Catalog No.:BCN5855
CAS No.:103917-26-6
- Nodosin
Catalog No.:BCN5854
CAS No.:10391-09-0
- 8alpha-Methacryloyloxybalchanin
Catalog No.:BCN4756
CAS No.:104021-39-8
- Trospium chloride
Catalog No.:BCC4582
CAS No.:10405-02-4
- LP533401 hcl
Catalog No.:BCC6377
CAS No.:1040526-12-2
- Quinovic acid 3-O-alpha-L-rhamnopyranoside
Catalog No.:BCN1636
CAS No.:104055-76-7
- Dihydroobovatin
Catalog No.:BCN3982
CAS No.:104055-79-0
- 3',5'-Diprenylgenistein
Catalog No.:BCN3572
CAS No.:104055-80-3
- Rehmapicroside
Catalog No.:BCN2884
CAS No.:104056-82-8
- Cyclo(Ile-Val)
Catalog No.:BCN2410
CAS No.:104068-43-1
- Atipamezole hydrochloride
Catalog No.:BCC7521
CAS No.:104075-48-1
- Zolantidine dimaleate
Catalog No.:BCC6922
CAS No.:104076-39-3
- Fmoc-D-Glu(OtBu)-OH
Catalog No.:BCC3496
CAS No.:104091-08-9
- SR 95531 hydrobromide
Catalog No.:BCC6997
CAS No.:104104-50-9
Suppression of inducible nitric oxide synthase and cyclooxygenase-2 expression by tussilagone from Farfarae flos in BV-2 microglial cells.[Pubmed:18481023]
Arch Pharm Res. 2008 May;31(5):645-52.
Activated microglia produce diverse neurotoxic factors such as nitric oxide (NO) and prostaglandin E(2) (PGE(2)) that may cause neurodegenerative diseases, including Alzheimer's disease and Parkinson's disease. From the EtOAc soluble fraction of Farfarae flos (Tussilago farfara), we purified Tussilagone as a bioactive compound by monitoring the inhibitory potential of NO production in activated microglia through the purification procedures. Tussilagone showed dose-dependent inhibition of NO and PGE(2) production in LPS-activated microglia with IC(50) values of 8.67 microM and 14.1 microM, respectively. It suppressed the expression of protein and mRNA of inducible nitric oxide synthase and cyclooxygenase-2 through the inhibition of 1-kappaBalpha degradation and nuclear translocation of p65 subunit of NF-kappaB. Therefore Tussilagone from Farfarae flos may have therapeutic potential in the treatment of neuro-inflammatory diseases through the inhibition of overproduction of NO and PGE(2).
The anti-inflammatory effect of tussilagone, from Tussilago farfara, is mediated by the induction of heme oxygenase-1 in murine macrophages.[Pubmed:19800419]
Int Immunopharmacol. 2009 Dec;9(13-14):1578-84.
Tussilagone (TSL), isolated from the flower of buds of Tussilago farfara (Compositae), is a sesquiterpenoid that is known to exert a variety of pharmacological activities. In the present study, we demonstrated that TSL exerts anti-inflammatory activities in murine macrophages by inducing heme oxygenase-1 (HO-1) expression. Treatment of RAW264.7 cells with TSL-induced HO-1 protein expression in a dose- and time-dependent manner without the induction of HO-1 mRNA expression. TSL-mediated HO-1 protein induction was not inhibited by treatment with actinomycin D, a transcriptional inhibitor, but by cycloheximide, a translational inhibitor. Moreover, mitogen-activated protein kinases (MAPKs) inhibitors such as SB203580, SP600125, and U0126 did not block TSL-mediated HO-1 protein expression, suggesting that the TSL-mediated HO induction may be regulated at the translational level. Consistent with the notion that HO-1 has anti-inflammatory properties, TSL inhibited the production of nitric oxide (NO), tumor necrosis factor (TNF)-alpha, and prostaglandin E2 (PGE2) as well as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression in lipopolysaccharide (LPS)-stimulated RAW264.7 cells and murine peritoneal macrophages. Inhibition of HO-1 activity by treatment with zinc protoporphyrin IX (ZnPP), a specific HO-1 inhibitor, abrogated the inhibitory effects of TSL on the production of NO and PGE2 in LPS-stimulated RAW264.7 cells. Taken together, TSL may be an effective HO-1 inducer that has anti-inflammatory effects, and a valuable compound for modulating inflammatory conditions.
Tussilagone inhibits dendritic cell functions via induction of heme oxygenase-1.[Pubmed:25091622]
Int Immunopharmacol. 2014 Oct;22(2):400-8.
Sesquiterpenoid Tussilagone (TUS) has a variety of pharmacological activities, such as anti-oxidant, anti-cancer, and anti-inflammatory activities. In this study, we investigated the effects of TUS on dendritic cell (DC) functions and the underlying mechanisms. TUS inhibited lipopolysaccharide (LPS)-induced activation of DCs, as shown by decrease in surface molecule expression, cytokine production, cell migration, and allo-T cell activation. In addition, TUS inhibited LPS-induced activation of NF-kappaB, MAPKs, and IRF-3 signalings in DCs, although it did not directly affect kinase activities of IRAK1/4, TAK1, and IKK, which suggests that TUS might indirectly inhibit TLR signaling in DCs. As a critical mechanism, we showed that TUS activated heme oxygenase-1 (HO-1), which degrades heme to immunosuppressive products, such as carbon monoxide and bilirubin. HO-1 inhibitor reversed the inhibitory activity of TUS in DCs. In conclusion, this study suggests that TUS inhibits DC function through the induction of HO-1.
Tussilagone suppresses colon cancer cell proliferation by promoting the degradation of beta-catenin.[Pubmed:24269588]
Biochem Biophys Res Commun. 2014 Jan 3;443(1):132-7.
Abnormal activation of the Wnt/beta-catenin signaling pathway frequently induces colon cancer progression. In the present study, we identified Tussilagone (TSL), a compound isolated from the flower buds of Tussilago farfara, as an inhibitor on beta-catenin dependent Wnt pathway. TSL suppressed beta-catenin/T-cell factor transcriptional activity and down-regulated beta-catenin level both in cytoplasm and nuclei of HEK293 reporter cells when they were stimulated by Wnt3a or activated by an inhibitor of glycogen synthase kinase-3beta. Since the mRNA level was not changed by TSL, proteasomal degradation might be responsible for the decreased level of beta-catenin. In SW480 and HCT116 colon cancer cell lines, TSL suppressed the beta-catenin activity and also decreased the expression of cyclin D1 and c-myc, representative target genes of the Wnt/beta-catenin signaling pathway, and consequently inhibited the proliferation of colon cancer cells. Taken together, TSL might be a potential chemotherapeutic agent for the prevention and treatment of human colon cancer.


