3',5'-DiprenylgenisteinCAS# 104055-80-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104055-80-3 | SDF | Download SDF |
| PubChem ID | 44257287 | Appearance | Yellow powder |
| Formula | C25H26O5 | M.Wt | 406.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
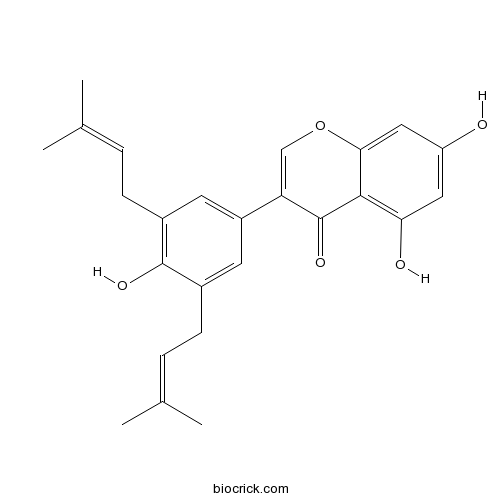
| Chemical Name | 5,7-dihydroxy-3-[4-hydroxy-3,5-bis(3-methylbut-2-enyl)phenyl]chromen-4-one | ||
| SMILES | CC(=CCC1=CC(=CC(=C1O)CC=C(C)C)C2=COC3=CC(=CC(=C3C2=O)O)O)C | ||
| Standard InChIKey | BGFLPCCZHOCCKB-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 3',5'-Diprenylgenistein inhibits protein tyrosine phosphatase 1B (PTP1B) activities, it can reduce muscle cell viability. 2. 3',5'-Diprenylgenistein exhibits significant glucose-uptake activity in basal and insulin-stimulated L6 myotubes. |
| Targets | AMPK | GLUT |

3',5'-Diprenylgenistein Dilution Calculator

3',5'-Diprenylgenistein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.46 mL | 12.3001 mL | 24.6002 mL | 49.2005 mL | 61.5006 mL |
| 5 mM | 0.492 mL | 2.46 mL | 4.92 mL | 9.8401 mL | 12.3001 mL |
| 10 mM | 0.246 mL | 1.23 mL | 2.46 mL | 4.92 mL | 6.1501 mL |
| 50 mM | 0.0492 mL | 0.246 mL | 0.492 mL | 0.984 mL | 1.23 mL |
| 100 mM | 0.0246 mL | 0.123 mL | 0.246 mL | 0.492 mL | 0.615 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydroobovatin
Catalog No.:BCN3982
CAS No.:104055-79-0
- Quinovic acid 3-O-alpha-L-rhamnopyranoside
Catalog No.:BCN1636
CAS No.:104055-76-7
- LP533401 hcl
Catalog No.:BCC6377
CAS No.:1040526-12-2
- Trospium chloride
Catalog No.:BCC4582
CAS No.:10405-02-4
- 8alpha-Methacryloyloxybalchanin
Catalog No.:BCN4756
CAS No.:104021-39-8
- Tussilagone
Catalog No.:BCN2770
CAS No.:104012-37-5
- Cinnamaldehyde
Catalog No.:BCN6241
CAS No.:104-55-2
- Cinnamyl alcohol
Catalog No.:BCN4967
CAS No.:104-54-1
- Anethole
Catalog No.:BCN5373
CAS No.:104-46-1
- 4-Methoxyphenylacetic acid
Catalog No.:BCN8467
CAS No.:104-01-8
- 4'-Hydroxypiptocarphin A
Catalog No.:BCN7113
CAS No.:103994-39-4
- 13-Hydroxygermacrone
Catalog No.:BCN3556
CAS No.:103994-29-2
- Rehmapicroside
Catalog No.:BCN2884
CAS No.:104056-82-8
- Cyclo(Ile-Val)
Catalog No.:BCN2410
CAS No.:104068-43-1
- Atipamezole hydrochloride
Catalog No.:BCC7521
CAS No.:104075-48-1
- Zolantidine dimaleate
Catalog No.:BCC6922
CAS No.:104076-39-3
- Fmoc-D-Glu(OtBu)-OH
Catalog No.:BCC3496
CAS No.:104091-08-9
- SR 95531 hydrobromide
Catalog No.:BCC6997
CAS No.:104104-50-9
- Kadsuric acid 3-methylester
Catalog No.:BCN3186
CAS No.:1041070-16-9
- Phellodendrine chloride
Catalog No.:BCN5934
CAS No.:104112-82-5
- Eldecalcitol
Catalog No.:BCC1548
CAS No.:104121-92-8
- Peramivir Trihydrate
Catalog No.:BCC4956
CAS No.:1041434-82-5
- Kuguaglycoside C
Catalog No.:BCN3276
CAS No.:1041631-93-9
- MitoPY1
Catalog No.:BCC6177
CAS No.:1041634-69-8
Genistein-derivatives from Tetracera scandens stimulate glucose-uptake in L6 myotubes.[Pubmed:19252305]
Biol Pharm Bull. 2009 Mar;32(3):504-8.
An EtOAc-soluble partition of the MeOH extract of a branch of Tetracera scandens (Dilleniaceae family) was subjected to a glucose-uptake assay, which led to the isolation and identification of five isoflavones of previously known structure namely, genistein (1), its derivatives 3',5'-diprenylgenistein (2), 6,8-diprenylgenistein (3), derrone (4) and alpinumisoflavone (5). Of these, compounds 2--5 exhibited significant glucose-uptake activity in basal and insulin-stimulated L6 myotubes. The findings from adenosine monophosphate-activated kinase (AMPK) activation and glucose transport protein4 (GLUT4) and GLUT1 over-expression revealed certain characteristics of compounds 2--5. These compounds inhibited protein tyrosine phosphatase 1B (PTP1B) activities with IC50 values ranging from 20.63 +/- 0.17 to 37.52 +/- 0.31 microM. No muscle cell toxicity was reported with compounds 3--5, while compounds 1 and 2 reduced muscle cell viability with IC50 values of 34.27 +/- 0.35 and 18.69 +/- 0.19 microM, respectively. It was concluded that T. scandens and its constituents exerted highly desirable activities on type 2 diabetes mellitus treatment since they significantly stimulated the uptake of glucose, AMPK phosphorylation, GLUT4 and GLUT1 mRNA expressions and PTP1B inhibition in L6 myotubes.


