CinnamaldehydeCAS# 104-55-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104-55-2 | SDF | Download SDF |
| PubChem ID | 637511 | Appearance | Oil |
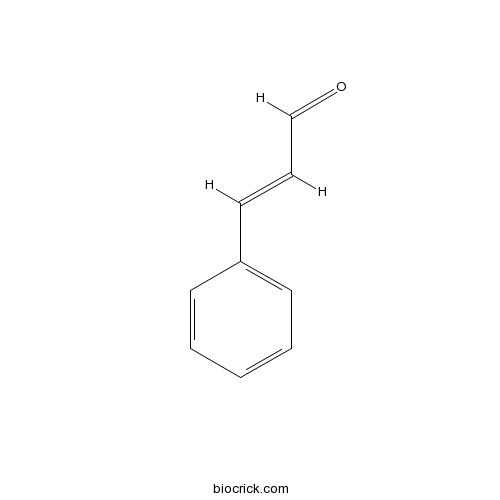
| Formula | C9H8O | M.Wt | 132.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | Cinnamic aldehyde;14371-10-9 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-phenylprop-2-enal | ||
| SMILES | C1=CC=C(C=C1)C=CC=O | ||
| Standard InChIKey | KJPRLNWUNMBNBZ-QPJJXVBHSA-N | ||
| Standard InChI | InChI=1S/C9H8O/c10-8-4-7-9-5-2-1-3-6-9/h1-8H/b7-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cinnamaldehyde has vasodilator, anti-melanoma, hypoglycemic, hypolipidemic, and anticancer effects, it possesses anti-bacterial activity against both gram-positive and gram-negative bacteria. Cinnamaldehyde has toxicity and antifeedant activities against the grain storage insects, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Cinnamaldehyde inhibited the expression of VEGF and HIF-α.Cinnamic aldehyde, a COX-2 inhibitor, exhibits cardioprotective, antidepressant-like, anti-leukemia, anti-oxidative and anti-inflammatory properties. Its supplementation can improve glucose and lipid homeostasis in diabetic animals. |
| Targets | NOS | Calcium Channel | VEGFR | HIF | ATPaseC | OX | PGE | TNF-α | IL Receptor | NO | ATPase | SOD |
| In vitro | Mechanisms of Bactericidal Action of Cinnamaldehyde against Listeria monocytogenes and of Eugenol against L. monocytogenes and Lactobacillus sakei[Reference: WebLink]Appl. Environ. Microb., 2004, 70(10):5750-5.The spice oil components eugenol and Cinnamaldehyde possess activity against both gram-positive and gram-negative bacteria, but the mechanisms of action remain obscure. In broth media at 20°C, 5 mM eugenol or 30 mM Cinnamaldehyde was bactericidal (>1-log reduction in the number of CFU per milliliter in 1 h) to Listeria monocytogenes. At a concentration of 6 mM eugenol was bactericidal to Lactobacillus sakei, but treatment with 0.5 M Cinnamaldehyde had no significant effect.
Toxicity and antifeedant activities of cinnamaldehyde against the grain storage insects, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch.[Reference: WebLink]J. Stored Prod. Res., 1998, 34(34):11-7.A methylene chloride extract of the spice, cinnamon, Cinnamomum aromaticum Nees, was shown to be insecticidal to Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch.
Mechanism of cinnamic aldehyde-inducing apoptosis of chronic myeloid leukemic cells in vitro.[Pubmed: 21729535]Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011 Jun;19(3):617-20.The aim of this study was to investigate the apoptosis-inducing effect of Cinnamic aldehyde (CA) on chronic myeloid leukemic (CML) cells and its mechanism. Potent nematicidal activity of phthalaldehyde, salicylaldehyde, and cinnamic aldehyde against Meloidogyne incognita.[Pubmed: 23379671]J Agric Food Chem. 2013 Feb 27;61(8):1794-803.
|
| In vivo | Cinnamaldehyde--a potential antidiabetic agent.[Pubmed: 17140783 ]Phytomedicine. 2007 Jan;14(1):15-22.Cinnamonum zeylanicum (cinnamon) is widely used in traditional system of medicine to treat diabetes in India.
Cinnamic aldehyde treatment alleviates chronic unexpected stress-induced depressive-like behaviors via targeting cyclooxygenase-2 in mid-aged rats.[Pubmed: 25556926]J Ethnopharmacol. 2015 Mar 13;162:97-103. COX-2 has been considered as a potent molecular target for prevention and therapy of depression. However, a recent study showed that COX-2 inhibitor does not improve depressive symptoms in persons aged 70 and over. Therefore, whether treatments targeting COX-2 have a clinical efficacy in depression, especially elderly individuals, remains unclear. Cinnamic aldehyde is a major constituent of Cinnamomum cassia, which has exhibited excellent anti-inflammatory activities as a COX-2 inhibitor. To investigate the potential antidepressant effect of Cinnamic aldehyde in mid-aged rats. |
| Kinase Assay | Cinnamaldehyde and cinnamaldehyde-containing micelles induce relaxation of isolated porcine coronary arteries: role of nitric oxide and calcium.[Pubmed: 24904214]Int J Nanomedicine. 2014 May 21;9:2557-66.Cinnamaldehyde, a major component of cinnamon, induces the generation of reactive oxygen species and exerts vasodilator and anticancer effects, but its short half-life limits its clinical use. The present experiments were designed to compare the acute relaxing properties of Cinnamaldehyde with those of self-assembling polymer micelles either loaded with Cinnamaldehyde or consisting of a polymeric prodrug [poly(Cinnamaldehyde)] that incorporates the compound in its backbone.
|
| Cell Research | Cinnamaldehyde/chemotherapeutic agents interaction and drug-metabolizing genes in colorectal cancer.[Pubmed: 24276478][Inhibitory effect of cinnamaldehyde on invasion capacities of human breast cancer cell line MDA-MB-435S and its relation with regulating the expression of miR-27a].[Pubmed: 25223182]Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014 Aug;34(8):964-9.To explore the inhibitory effect of Cinnamaldehyde on invasion capacities of human breast cancer cell line MDA-MB-435S and its relation with regulating the expression of miR-27a.
Mol Med Rep. 2014 Feb;9(2):669-76.Cinnamaldehyde is an active monomer isolated from the stem bark of Cinnamomum cassia, a traditional oriental medicinal herb, which is known to possess marked antitumor effects in vitro and in vivo.
|
| Animal Research | Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats.[Pubmed: 24001892]Research on tumorigenicity of cinnamaldehyde in melanoma cell lines and its mechanism.[Pubmed: 24643680]Tumour Biol. 2014 Jun;35(6):5717-22.Melanoma is a highly malignant tumor originating from melanocytes. This disease is characterized by inconspicuous onset, high malignancy, and poor prognosis.
J Ethnopharmacol. 2013 Oct 28;150(1):125-30.Cinnamomum cassia is a well-known traditional Chinese herb that is widely used for the treatment of ischemic heart disease (IHD). It has favorable effects, but its mechanism is not clear. To investigate the effects of Cinnamic aldehyde (CA) and cinnamic acid (CD) isolated from Cinnamomum cassia against myocardial ischemia produced in rats by isoproterenol (ISO). |

Cinnamaldehyde Dilution Calculator

Cinnamaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.5643 mL | 37.8215 mL | 75.643 mL | 151.2859 mL | 189.1074 mL |
| 5 mM | 1.5129 mL | 7.5643 mL | 15.1286 mL | 30.2572 mL | 37.8215 mL |
| 10 mM | 0.7564 mL | 3.7821 mL | 7.5643 mL | 15.1286 mL | 18.9107 mL |
| 50 mM | 0.1513 mL | 0.7564 mL | 1.5129 mL | 3.0257 mL | 3.7821 mL |
| 100 mM | 0.0756 mL | 0.3782 mL | 0.7564 mL | 1.5129 mL | 1.8911 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cinnamyl alcohol
Catalog No.:BCN4967
CAS No.:104-54-1
- Anethole
Catalog No.:BCN5373
CAS No.:104-46-1
- 4-Methoxyphenylacetic acid
Catalog No.:BCN8467
CAS No.:104-01-8
- 4'-Hydroxypiptocarphin A
Catalog No.:BCN7113
CAS No.:103994-39-4
- 13-Hydroxygermacrone
Catalog No.:BCN3556
CAS No.:103994-29-2
- Ceftiofur hydrochloride
Catalog No.:BCC8911
CAS No.:103980-44-5
- Esculentic acid
Catalog No.:BCN5856
CAS No.:103974-74-9
- 15-Nor-14-oxolabda-8(17),12-dien-18-oic acid
Catalog No.:BCN1637
CAS No.:1039673-32-9
- Oleanolic acid 3-O-beta-D-glucosyl-(1->3)-alpha-L-rhamnosyl(1->2)-alpha-L-arabinoside
Catalog No.:BCN8132
CAS No.:103956-33-8
- Lupeol caffeate
Catalog No.:BCN5855
CAS No.:103917-26-6
- Nodosin
Catalog No.:BCN5854
CAS No.:10391-09-0
- (-)-Isodocarpin
Catalog No.:BCN3280
CAS No.:10391-08-9
- Tussilagone
Catalog No.:BCN2770
CAS No.:104012-37-5
- 8alpha-Methacryloyloxybalchanin
Catalog No.:BCN4756
CAS No.:104021-39-8
- Trospium chloride
Catalog No.:BCC4582
CAS No.:10405-02-4
- LP533401 hcl
Catalog No.:BCC6377
CAS No.:1040526-12-2
- Quinovic acid 3-O-alpha-L-rhamnopyranoside
Catalog No.:BCN1636
CAS No.:104055-76-7
- Dihydroobovatin
Catalog No.:BCN3982
CAS No.:104055-79-0
- 3',5'-Diprenylgenistein
Catalog No.:BCN3572
CAS No.:104055-80-3
- Rehmapicroside
Catalog No.:BCN2884
CAS No.:104056-82-8
- Cyclo(Ile-Val)
Catalog No.:BCN2410
CAS No.:104068-43-1
- Atipamezole hydrochloride
Catalog No.:BCC7521
CAS No.:104075-48-1
- Zolantidine dimaleate
Catalog No.:BCC6922
CAS No.:104076-39-3
- Fmoc-D-Glu(OtBu)-OH
Catalog No.:BCC3496
CAS No.:104091-08-9
Potent nematicidal activity of phthalaldehyde, salicylaldehyde, and cinnamic aldehyde against Meloidogyne incognita.[Pubmed:23379671]
J Agric Food Chem. 2013 Feb 27;61(8):1794-803.
The nematicidal activity of selected aromatic aldehydes was tested against the root knot nematode Meloidogyne incognita. The most active aldehyde was phthalaldehyde (1) with an EC(50) value of 11 +/- 6 mg/L followed by salicylaldehyde (2) and cinnamic aldehyde (3) with EC(50) values of 11 +/- 1 and 12 +/- 5 mg/L, respectively. On the other hand, structurally related aldehydes such as 2-methoxybenzaldehyde (21), 3,4-dimethoxybenzaldehyde, and vanillin (23) were not active at the concentration of 1000 mg/L. By liquid chromatography-mass spectrometry the reactivity of tested aldehydes against a synthetic peptide resembling the nematode cuticle was characterized. At the test concentration of 1 mM, the main adduct formation was observed for 3,4-dihydroxybenzaldehyde (22), 2-methoxybenzaldehyde (21), and 3,4-dimethoxybenzaldehyde. Considering that 2-methoxybenzaldehyde (21) and 3,4-dimethoxybenzaldehyde were not active against M. incognita in in vitro experiments led us to hypothesize a different mechanism of action rather than an effect on the external cuticle modification of nematodes. When the toxicity of the V-ATPase inhibitor pyocyanin (10) was tested against M. incognita J2 nematodes, an EC(50) at 24 h of 72 +/- 25 mg/L was found. The redox-active compounds such as phthalaldehyde (1) and salicylaldehyde (2) may share a common mode of action inhibiting nematode V-ATPase enzyme. The results of this investigation reveal that aromatic redox-active aldehydes can be considered as potent nematicides, and further investigation is needed to completely clarify their mode of action.
[Mechanism of cinnamic aldehyde-inducing apoptosis of chronic myeloid leukemic cells in vitro].[Pubmed:21729535]
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011 Jun;19(3):617-20.
The aim of this study was to investigate the apoptosis-inducing effect of cinnamic aldehyde (CA) on chronic myeloid leukemic (CML) cells and its mechanism. K562 cells and primary bone marrow mononuclear cells (MNC) from patients with CML were treated by various concentrations of CA. Flow cytometry was employed to measure the apoptosis of K562 cells and primary CML bone marrow MNC. Western blot was used to determine the expression of C-MYC and the phosphorylation of CrkL in K562 cells, and real-time polymerase chain reaction (real-time PCR) was used to quantify the expression of BCR-ABL mRNA in K562 cells. The results indicated that CA induced the apoptosis of K562 cells in a time- and dose-dependent manner. CA induced apoptosis of CML MNC dose-dependently. CA inhibited the expression of BCR-ABL mRNA and C-MYC, reduced CrkL phosphorylation levels in K562 cells. It is concluded that CA induces apoptosis of CML cells in vitro. Down-regulation of the expression and function of BCR-ABL may be one of its most important anti-leukemia mechanisms.
Cinnamaldehyde/chemotherapeutic agents interaction and drug-metabolizing genes in colorectal cancer.[Pubmed:24276478]
Mol Med Rep. 2014 Feb;9(2):669-76.
Cinnamaldehyde is an active monomer isolated from the stem bark of Cinnamomum cassia, a traditional oriental medicinal herb, which is known to possess marked antitumor effects in vitro and in vivo. The aim of the present study was to examine the potential advantages of using Cinnamaldehyde in combination with chemotherapeutic agents commonly used in colorectal carcinoma (CRC) therapy, as well as to investigate the effect of Cinnamaldehyde on chemotherapeutic-associated gene expression. The synergistic interaction of Cinnamaldehyde and chemotherapeutic agents on human CRC HT-29 and LoVo cells was evaluated using the combination index (CI) method. The double staining with Annexin V conjugated to fluorescein-isothiocyanate and phosphatidylserine was employed for apoptosis detection. The expression of drug-metabolizing genes, including excision repair crosscomplementing 1 (ERCC1), orotate phosphoribosyltransferase (OPRT), thymidylate synthase (TS), breast cancer susceptibility gene 1 (BRCA1) and topoisomerase 1 (TOPO1), all in HT-29 and LoVo cells, with or without the addition of Cinnamaldehyde, was examined by quantitative polymerase chain reaction (PCR). Cinnamaldehyde had a synergistic effect on the chemotherapeutic agents cytotoxicity in HT-29 and LoVo cells. In addition, Cinnamaldehyde suppressed BRCA1, TOPO1, ERCC1 and TS mRNA expression, except for OPRT expression, which was markedly upregulated. Our findings indicate that Cinnamaldehyde appears to be a promising candidate as an adjuvant in combination therapy with 5-fluorouracil (5-FU) and oxaliplatin (OXA), two chemotherapeutic agents used in CRC treatment. The possible mechanisms of its action may involve the regulation of drugmetabolizing genes.
[Inhibitory effect of cinnamaldehyde on invasion capacities of human breast cancer cell line MDA-MB-435S and its relation with regulating the expression of miR-27a].[Pubmed:25223182]
Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014 Aug;34(8):964-9.
OBJECTIVE: To explore the inhibitory effect of Cinnamaldehyde on invasion capacities of human breast cancer cell line MDA-MB-435S and its relation with regulating the expression of miR-27a. METHODS: The effect of Cinnamaldehyde on invasive capacities of MDA-MB-435S was measured by Transwell matrigel invasion assay. The effect of miR-27a expression on invasive capabilities of MDA-MB-435S, the intervention of Cinnamaldehyde in the miR-27a expression, and its relation with its effect on invasive capabilities were defected with liposome 2000 transinfection miRNA27a mimics/inhibitors, real time-polymerase chain reaction (Real-time PCR), and Transwell chamber model. RESULTS: Compared with the control group, the number of cells passing through the transwell chamber was more significantly reduced after treated by Cinnamaldehyde for 12 h (P < 0.05). The miR-27a expression was 962.07 times and 40% of that of the control group after transinfected by miR-27a mimics and miR-27a inhibitors. After transinfected by miR-27a inhibitors, the number of cells passing through the transwell chamber was more significantly reduced (P < 0.05). The miR-27a expression of MDA-MB-435S was down-regulated by 12-h treatment of Cinnamaldehyde (2(-deltaCt) = 0.56, 0.18, 0.18, respectively). The number of miR-27a mimics transinfection pretreated MDA-MB-435S cells passing through the transwell chamber increased more obviously than the number of un-pretreated MDA-MB-435S cells in the control group (P < 0.05). CONCLUSIONS: Cinnamaldehyde could inhibit invasive capabilities of human breast cancer cell line MDA-MB-435S. The over-expression of miR-27a played an important role in the invasive capability of MDA-MB-435S. The inhibition of Cinnamaldehyde on invasive capabilities of MDA-MB-435S cells was correlated with down-regulating the expression of miR-27a.
Cinnamaldehyde and cinnamaldehyde-containing micelles induce relaxation of isolated porcine coronary arteries: role of nitric oxide and calcium.[Pubmed:24904214]
Int J Nanomedicine. 2014 May 21;9:2557-66.
BACKGROUND AND PURPOSE: Cinnamaldehyde, a major component of cinnamon, induces the generation of reactive oxygen species and exerts vasodilator and anticancer effects, but its short half-life limits its clinical use. The present experiments were designed to compare the acute relaxing properties of Cinnamaldehyde with those of self-assembling polymer micelles either loaded with Cinnamaldehyde or consisting of a polymeric prodrug [poly(Cinnamaldehyde)] that incorporates the compound in its backbone. METHODS: Rings of porcine coronary arteries were contracted with the thromboxane A2 receptor agonist U46619 or 40 mM KCl, and changes in isometric tension were recorded. RESULTS: Cinnamaldehyde induced concentration-dependent but endothelium-independent, nitric oxide synthase (NOS)-independent, cyclooxygenase-independent, soluble guanylyl cyclase (sGC)-independent, calcium-activated potassium-independent, and TRPA1 channel-independent relaxations. Cinnamaldehyde also inhibited the contractions induced by 40 mM KCl Ca(2+) reintroduction in 40 mM KCl Ca(2+)-free solution or by the Ca(2+) channel opener Bay K8644. Cinnamaldehyde-loaded control micelles induced complete, partly endothelium-dependent relaxations sensitive to catalase and inhibitors of NOS or sGC, but not cyclooxygenase or TRPA1, channels. Cinnamaldehyde-loaded micelles also inhibited contractions induced by 40 mM KCl Ca(2+) reintroduction or Bay K8644. Poly(Cinnamaldehyde) micelles induced only partial, endothelium-dependent relaxations that were reduced by inhibitors of NOS or sGC and by catalase and the antioxidant tiron, but not by indomethacin or TRPA1 channel blockers. CONCLUSION: The present findings demonstrate that Cinnamaldehyde-loaded and poly(Cinnamaldehyde) micelles possess vasodilator properties, but that the mechanism underlying the relaxation that they cause differs from that of Cinnamaldehyde, and thus could be used both to relieve coronary vasospasm and for therapeutic drug delivery.
Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats.[Pubmed:24001892]
J Ethnopharmacol. 2013 Oct 28;150(1):125-30.
ETHNOPHARMACOLOGICAL RELEVANCE: Cinnamomum cassia is a well-known traditional Chinese herb that is widely used for the treatment of ischemic heart disease (IHD). It has favorable effects, but its mechanism is not clear. To investigate the effects of cinnamic aldehyde (CA) and cinnamic acid (CD) isolated from Cinnamomum cassia against myocardial ischemia produced in rats by isoproterenol (ISO). MATERIALS AND METHODS: Ninety male Sprague-Dawley rats were randomized equally to nine groups: a control group, an untreated model group, CA (22.5, 45, 90 mg/kg) or CD (37.5, 75, 150 mg/kg) treatment, or propranolol (30 mg/kg). Rats were treated for 14 days and then given ISO, 4 mg/kg for 2 consecutive days by subcutaneous injection. ST-segment elevation was measured after the last administration. Serum levels of creatine kinase (CK), lactate dehydrogenase (LDH), tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), nitric oxide (NO), and blood rheology were measured after the rats were sacrificed. The hearts were excised for determining heart weight index, microscopic examination, superoxide dismutase (SOD) and malondialdehyde (MDA) measurements. RESULTS: CA and CD decreased the ST elevation induced by acute myocardial ischemia, decreased serum levels of CK-MB, LDH, TNF-alpha and IL-6, and increased serum NO activity. CA and CD increased SOD activity and decreased MDA content in myocardial tissue. CONCLUSION: CA and CD were cardioprotective in a rat model of ischemic myocardial injury. The protection was attributable to anti-oxidative and anti-inflammatory properties, as well as increased NO. The results support further study of CA and CD as potential treatments for ischemic heart disease.
Cinnamic aldehyde treatment alleviates chronic unexpected stress-induced depressive-like behaviors via targeting cyclooxygenase-2 in mid-aged rats.[Pubmed:25556926]
J Ethnopharmacol. 2015 Mar 13;162:97-103.
ETHNOPHARMACOLOGICAL RELEVANCE: COX-2 has been considered as a potent molecular target for prevention and therapy of depression. However, a recent study showed that COX-2 inhibitor does not improve depressive symptoms in persons aged 70 and over. Therefore, whether treatments targeting COX-2 have a clinical efficacy in depression, especially elderly individuals, remains unclear. Cinnamic aldehyde is a major constituent of Cinnamomum cassia, which has exhibited excellent anti-inflammatory activities as a COX-2 inhibitor. To investigate the potential antidepressant effect of cinnamic aldehyde in mid-aged rats. MATERIALS AND METHODS: The depressive-like behaviors were measured after the rats exposed to chronic unexpected mild stress (CUMS). Cinnamic aldehyde was administrated by oral gavage to stressed rats (22.5, 45, 90 mg/kg, respectively) for 21 days. The mRNA, protein expression and activity of cyclooxygenase-2 (COX-2), as well as prostaglandin E2 (PGE2) levels were measured in the frontal cortex and hippocampus of stressed animals. RESULTS: We found that CUMS procedure not only decreased the sucrose preference, but also elevated the COX-2 activity, mRNA and protein levels, and increased PGE2 concentration in rat brain regions. Treatment with high doses of cinnamic aldehyde (45, 90 mg/kg) reversed the behavioral abnormalities, and decreased the COX-2 protein and activity (but not COX-2 mRNA expression) and PGE2 concentration in frontal cortex and hippocampus of stressed rats. CONCLUSION: Cinnamic aldehyde exerted antidepressant-like effects in stressed mid-aged rats, and its mechanism of action appears to decrease COX-2 protein and activity. The current findings suggest that targeting COX-2 system might be benefit to the depression, especially elderly individuals and cinnamic aldehyde might be a promising medicine to treat the subjects in the depression.
Research on tumorigenicity of cinnamaldehyde in melanoma cell lines and its mechanism.[Pubmed:24643680]
Tumour Biol. 2014 Jun;35(6):5717-22.
Melanoma is a highly malignant tumor originating from melanocytes. This disease is characterized by inconspicuous onset, high malignancy, and poor prognosis. The aim of this study is to explore the effect of Cinnamaldehyde on melanoma tumorigenicity and its mechanism. Melanoma cells were subcutaneously injected into a nude mouse to establish the tumour model. A comparison was made for the difference in formation and growth of melanoma cell tumor between normal saline and Cinnamaldehyde. A comparison was also made for the number of new vessels between the normal saline group (the control group) and the Cinnamaldehyde group (the experimental group) through immumohistochemical staining. The western blot was used to detect the difference in expression levels of vascularization related proteins. The results indicated that the volume of tumors formed and the number of new vessels in melanoma cells of the Cinnamaldehyde group decreased significantly compared with those in the cells of the normal saline group. A further study indicated that the expression of hypoxia-inducible factor-a (HIF-alpha) and vascular endothelial growth factor (VEGF) in the melanoma of the Cinnamaldehyde group decreased significantly. In conclusion, Cinnamaldehyde plays a certain role in inhibiting the occurrence and progression of melanoma and its action mechanism may be manifested by inhibiting expression of VEGF and HIF-alpha, thus blood vessel simulation and formation of new blood vessels of melanoma cells, and growth of tumors accordingly.
Cinnamaldehyde--a potential antidiabetic agent.[Pubmed:17140783]
Phytomedicine. 2007 Jan;14(1):15-22.
Cinnamonum zeylanicum (cinnamon) is widely used in traditional system of medicine to treat diabetes in India. The present study was carried out to isolate and identify the putative antidiabetic compounds based on bioassay-guided fractionation; the compound identified decreased the plasma glucose levels. The active compound was purified by repeat column and structure of Cinnamaldehyde was determined on the basis of chemical and physiochemical evidence. The LD(50) value of Cinnamaldehyde was determined as 1850+/-37 mg/kg bw. Cinnamaldehyde was administered at different doses (5, 10 and 20 mg/kg bw) for 45 days to streptozotocin (STZ) (60 mg/kg bw)-induced male diabetic wistar rats. It was found that plasma glucose concentration was significantly (p<0.05) decreased in a dose-dependent manner (63.29%) compared to the control. In addition, oral administration of Cinnamaldehyde (20 mg/kg bw) significantly decreased glycosylated hemoglobin (HbA(1C)), serum total cholesterol, triglyceride levels and at the same time markedly increased plasma insulin, hepatic glycogen and high-density lipoprotein-cholesterol levels. Also Cinnamaldehyde restored the altered plasma enzyme (aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, alkaline phosphatase and acid phosphatase) levels to near normal. Administration of glibenclamide, a reference drug (0.6 mg/kg bw) also produced a significant (p<0.05) reduction in blood glucose concentration in STZ-induced diabetic rats. The results of this experimental study indicate that Cinnamaldehyde possesses hypoglycemic and hypolipidemic effects in STZ-induced diabetic rats.


