Atipamezole hydrochlorideCAS# 104075-48-1 |
- Saquinavir mesylate
Catalog No.:BCC1922
CAS No.:149845-06-7
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9
- Tipranavir
Catalog No.:BCC2002
CAS No.:174484-41-4
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104075-48-1 | SDF | Download SDF |
| PubChem ID | 13649426 | Appearance | Powder |
| Formula | C14H17ClN2 | M.Wt | 248.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 75 mg/mL (301.51 mM; Need ultrasonic and warming) DMSO : ≥ 47 mg/mL (188.94 mM) *"≥" means soluble, but saturation unknown. | ||
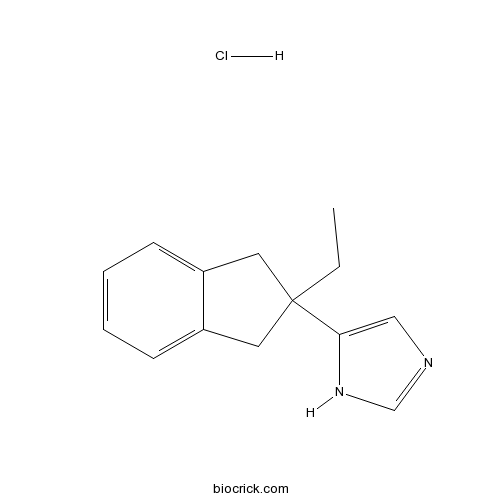
| Chemical Name | 5-(2-ethyl-1,3-dihydroinden-2-yl)-1H-imidazole;hydrochloride | ||
| SMILES | CCC1(CC2=CC=CC=C2C1)C3=CN=CN3.Cl | ||
| Standard InChIKey | PCCVCJAQMHDWJY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H16N2.ClH/c1-2-14(13-9-15-10-16-13)7-11-5-3-4-6-12(11)8-14;/h3-6,9-10H,2,7-8H2,1H3,(H,15,16);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective α2-adrenergic receptor antagonist (Ki values are 1.1, 1.0, 0.89, 1300 and 6500 nM for α2A, α2B, α2C, α1A and α1B receptors respectively). Brain penetrant. |

Atipamezole hydrochloride Dilution Calculator

Atipamezole hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0201 mL | 20.1005 mL | 40.201 mL | 80.402 mL | 100.5025 mL |
| 5 mM | 0.804 mL | 4.0201 mL | 8.0402 mL | 16.0804 mL | 20.1005 mL |
| 10 mM | 0.402 mL | 2.0101 mL | 4.0201 mL | 8.0402 mL | 10.0503 mL |
| 50 mM | 0.0804 mL | 0.402 mL | 0.804 mL | 1.608 mL | 2.0101 mL |
| 100 mM | 0.0402 mL | 0.201 mL | 0.402 mL | 0.804 mL | 1.005 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cyclo(Ile-Val)
Catalog No.:BCN2410
CAS No.:104068-43-1
- Rehmapicroside
Catalog No.:BCN2884
CAS No.:104056-82-8
- 3',5'-Diprenylgenistein
Catalog No.:BCN3572
CAS No.:104055-80-3
- Dihydroobovatin
Catalog No.:BCN3982
CAS No.:104055-79-0
- Quinovic acid 3-O-alpha-L-rhamnopyranoside
Catalog No.:BCN1636
CAS No.:104055-76-7
- LP533401 hcl
Catalog No.:BCC6377
CAS No.:1040526-12-2
- Trospium chloride
Catalog No.:BCC4582
CAS No.:10405-02-4
- 8alpha-Methacryloyloxybalchanin
Catalog No.:BCN4756
CAS No.:104021-39-8
- Tussilagone
Catalog No.:BCN2770
CAS No.:104012-37-5
- Cinnamaldehyde
Catalog No.:BCN6241
CAS No.:104-55-2
- Cinnamyl alcohol
Catalog No.:BCN4967
CAS No.:104-54-1
- Anethole
Catalog No.:BCN5373
CAS No.:104-46-1
- Zolantidine dimaleate
Catalog No.:BCC6922
CAS No.:104076-39-3
- Fmoc-D-Glu(OtBu)-OH
Catalog No.:BCC3496
CAS No.:104091-08-9
- SR 95531 hydrobromide
Catalog No.:BCC6997
CAS No.:104104-50-9
- Kadsuric acid 3-methylester
Catalog No.:BCN3186
CAS No.:1041070-16-9
- Phellodendrine chloride
Catalog No.:BCN5934
CAS No.:104112-82-5
- Eldecalcitol
Catalog No.:BCC1548
CAS No.:104121-92-8
- Peramivir Trihydrate
Catalog No.:BCC4956
CAS No.:1041434-82-5
- Kuguaglycoside C
Catalog No.:BCN3276
CAS No.:1041631-93-9
- MitoPY1
Catalog No.:BCC6177
CAS No.:1041634-69-8
- Alpinoid D
Catalog No.:BCN3593
CAS No.:1041740-13-9
- Stanozolol
Catalog No.:BCC9154
CAS No.:10418-03-8
- 10-Nitro-camptothecin
Catalog No.:BCN2581
CAS No.:104195-61-1
Sedative and cardiopulmonary effects of medetomidine hydrochloride and xylazine hydrochloride and their reversal with atipamezole hydrochloride in calves.[Pubmed:18312129]
Am J Vet Res. 2008 Mar;69(3):319-29.
OBJECTIVE: To assess the sedative and cardiopulmonary effects of medetomidine and xylazine and their reversal with atipamezole in calves. ANIMALS: 25 calves. PROCEDURES: A 2-phase (7-day interval) study was performed. Sedative characteristics (phase I) and cardiopulmonary effects (phase II) of medetomidine hydrochloride and xylazine hydrochloride administration followed by Atipamezole hydrochloride administration were evaluated. In both phases, calves were randomly allocated to receive 1 of 4 treatments IV: medetomidine (0.03 mg/kg) followed by atipamezole (0.1 mg/kg; n = 6), xylazine (0.3 mg/kg) followed by atipamezole (0.04 mg/kg; 7), medetomidine (0.03 mg/kg) followed by saline (0.9% NaCl; 6) solution (10 mL), and xylazine (0.3 mg/kg) followed by saline solution (10 mL; 6). Atipamezole or saline solution was administered 20 minutes after the first injection. Cardiopulmonary variables were recorded at intervals for 35 minutes after medetomidine or xylazine administration. RESULTS: At the doses evaluated, xylazine and medetomidine induced a similar degree of sedation in calves; however, the duration of medetomidine-associated sedation was longer. Compared with pretreatment values, heart rate, cardiac index, and PaO(2) decreased, whereas central venous pressure, PaCO(2), and pulmonary artery pressures increased with medetomidine or xylazine. Systemic arterial blood pressures and vascular resistance increased with medetomidine and decreased with xylazine. Atipamezole reversed the sedative and most of the cardiopulmonary effects of both drugs. CONCLUSIONS AND CLINICAL RELEVANCE: At these doses, xylazine and medetomidine induced similar degrees of sedation and cardiopulmonary depression in calves, although medetomidine administration resulted in increases in systemic arterial blood pressures. Atipamezole effectively reversed medetomidine- and xylazine-associated sedative and cardiopulmonary effects in calves.
Immobilisation of impala (Aepyceros melampus) with a ketamine hydrochloride/medetomidine hydrochloride combination, and reversal with atipamezole hydrochloride.[Pubmed:15214689]
J S Afr Vet Assoc. 2004 Mar;75(1):14-8.
A combination of medetomidine hydrochloride (medetomidine) and ketamine hydrochloride (ketamine) was evaluated in 16 boma-confined and 19 free-ranging impalas (Aepyceros melampus) to develop a non-opiate immobilisation protocol. In free-ranging impala a dose of 220 +/- 34 microg/kg medetomidine and 4.4 +/- 0.7 mg/kg ketamine combined with 7500 IU of hyaluronidase induced recumbency within 4.5 +/- 1.5 min, with good muscle relaxation, a stable heart rate and blood pH. PaCO2 was maintained within acceptable ranges. The animals were hypoxic with reduced oxygen saturation and low PaO2 in the presence of an elevated respiration rate, therefore methods for respiratory support are indicated. The depth of sedation was adequate for minor manipulations but additional anaesthesia is indicated for painful manipulations. Immobilisation was reversed by 467 +/- 108 microg/kg Atipamezole hydrochloride (atipamezole) intramuscularly, but re-sedation was observed several hours later, possibly due to a low atipamezole:medetomidine ratio of 2:1. Therefore, this immobilisation and reversal protocol would subject impalas to possible predation or conspecific aggression following reversal if they were released into the wild. If the protocol is used on free-ranging impala, an atipamezole:medetomidine ratio of 5:1 should probably be used to prevent re-sedation.
An evaluation of the influence of medetomidine hydrochloride and atipamezole hydrochloride on the arrhythmogenic dose of epinephrine in dogs during halothane anesthesia.[Pubmed:8825986]
Can J Vet Res. 1996 Jan;60(1):1-6.
Alterations in the arrhythmogenic dose of epinephrine (ADE) were determined following administration of medetomidine hydrochloride (750 micrograms/M2) and a saline placebo, or medetomidine hydrochloride (750 micrograms/M2), followed by specific medetomidine reversal agent, Atipamezole hydrochloride (50 micrograms/kg) 20 min later, in halothane-anesthetized dogs (n = 6). ADE determinations were made prior to the administration of either treatment, 20 min and 4 h following medetomidine/saline or medetomidine/atipamezole administration. Epinephrine was infused for 3 min at increasing dose rates (2.5 and 5.0 micrograms/kg/min) until the arrhythmia criterion (4 or more intermittent or continuous premature ventricular contractions) was reached. The interinfusion interval was 20 min. There were no significant differences in the amount of epinephrine required to reach the arrhythmia criterion following the administration of either treatment. In addition, the ADE at each determination was not different between treatment groups. In this study, the administration of medetomidine to halothane-anesthetized dogs did not alter their arrhythmogenic response to infused epinephrine.
Alpha2A adrenergic receptor activation inhibits epileptiform activity in the rat hippocampal CA3 region.[Pubmed:17341653]
Mol Pharmacol. 2007 Jun;71(6):1572-81.
Norepinephrine has potent antiepileptic properties, the pharmacology of which is unclear. Under conditions in which GABAergic inhibition is blocked, norepinephrine reduces hippocampal cornu ammonis 3 (CA3) epileptiform activity through alpha(2) adrenergic receptor (AR) activation on pyramidal cells. In this study, we investigated which alpha(2)AR subtype(s) mediates this effect. First, alpha(2)AR genomic expression patterns of 25 rat CA3 pyramidal cells were determined using real-time single-cell reverse transcription-polymerase chain reaction, demonstrating that 12 cells expressed alpha(2A)AR transcript; 3 of the 12 cells additionally expressed mRNA for alpha(2C)AR subtype and no cells possessing alpha(2B)AR mRNA. Hippocampal CA3 epileptiform activity was then examined using field potential recordings in brain slices. The selective alphaAR agonist 6-fluoronorepinephrine caused a reduction of CA3 epileptiform activity, as measured by decreased frequency of spontaneous epileptiform bursts. In the presence of betaAR blockade, concentration-response curves for AR agonists suggest that an alpha(2)AR mediates this response, as the rank order of potency was 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine (UK-14304) >or= epinephrine >6-fluoronorepinephrine > norepinephrine >>> phenylephrine. Finally, equilibrium dissociation constants (K(b)) of selective alphaAR antagonists were functionally determined to confirm the specific alpha(2)AR subtype inhibiting CA3 epileptiform activity. Apparent K(b) values calculated for atipamezole (1.7 nM), MK-912 (4.8 nM), BRL-44408 (15 nM), yohimbine (63 nM), ARC-239 (540 nM), prazosin (4900 nM), and terazosin (5000 nM) correlated best with affinities previously determined for the alpha(2A)AR subtype (r = 0.99, slope = 1.0). These results suggest that, under conditions of impaired GABAergic inhibition, activation of alpha(2A)ARs is primarily responsible for the antiepileptic actions of norepinephrine in the rat hippocampal CA3 region.
An alpha(2)-adrenergic antagonist, atipamezole, facilitates behavioral recovery after focal cerebral ischemia in rats.[Pubmed:11249969]
Neuropharmacology. 2001 Mar;40(4):597-606.
Previous studies suggest that enhanced noradrenergic neurotransmission promotes functional recovery following cerebral lesions. The present study investigated whether systemic administration of an alpha(2)-adrenergic antagonist, atipamezole, facilitates recovery following transient focal cerebral ischemia in rats. The effect of atipamezole therapy on recovery from ischemia was compared with the effect of enriched-environment housing in rats. Ischemia was induced by occlusion of the right middle cerebral artery (MCA) for 120 min using the intraluminal filament model. Daily atipamezole treatment (1 mg/kg, subcutaneously) was started on day 2 after ischemia induction and drug administration stopped after 10 days. Another group of rats was housed in an enriched environment from day 2 following ischemia induction until the end of the experiment. Several different behavioral tests were used to measure functional recovery during the 26 days following the induction of focal cerebral ischemia. There was improved performance in the limb-placing test from the beginning of atipamezole treatment to day 8, and in wheel-running in the foot-slip test on days 2 and 4. Enriched-environment housing facilitated recovery in the foot-slip test in a later phase of the test period (days 8 to 10). Discovery of a hidden platform in a water-maze task was also facilitated in rats housed in the enriched environment, but this was probably due to the increased swimming speed of these rats. The present data suggest that the alpha(2)-adrenergic antagonist, atipamezole, facilitates sensorimotor recovery after focal ischemia, but has no effect on subsequent water-maze tests assessing spatial learning and memory, when assessed 11 days after the cessation of drug administration.
Highly selective and specific antagonism of central and peripheral alpha 2-adrenoceptors by atipamezole.[Pubmed:2567152]
Arch Int Pharmacodyn Ther. 1989 Jan-Feb;297:190-204.
The potency, selectivity and specificity of atipamezole [MPV-1248, 4-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole], as an alpha 2-adrenoceptor antagonist was studied. In receptor binding studies [( 3H]-clonidine and [3H]-prazosin displacement) an alpha 2/alpha 1 selectivity ratio of 8526 was obtained for atipamezole, while idazoxan and yohimbine showed ratios of 27 and 40, respectively. Atipamezole had also about a 100 times higher affinity on alpha 2-adrenoceptors than the reference compounds. In the electrically stimulated prostatic portion of rat vas deferens, atipamezole showed potent competitive antagonistic activity against clonidine (pA2 8.6) and medetomidine (pA2 8.7) at presynaptic alpha 2-adrenoceptors. In the epididymal portion of the rat vas deferens, atipamezole had only weak competitive antagonistic activity against phenylephrine (pA2 5.0). In binding studies and studies with isolated organs, atipamezole had no effect on beta 1-, beta 2-, H1-, H2-, 5-HT1-, 5-HT2-, muscarine, DA2-, tryptamine, GABA, opiate and benzodiazepine receptors. In the pithed rat, atipamezole, like idazoxan, had partial alpha 1-adrenoceptor-mediated vasoconstrictor effects in addition to potent alpha 2-adrenoceptor blocking activity. In mice, medetomidine-induced sedation was effectively antagonized by atipamezole. These results show that atipamezole is a potent, selective and specific antagonist of both centrally and peripherally located alpha 2-adrenoceptors.


