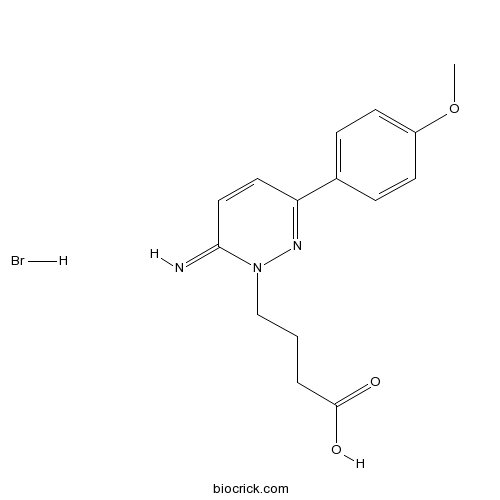
SR 95531 hydrobromideSelective, competitive GABAA receptor antagonist CAS# 104104-50-9 |
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- GW3965
Catalog No.:BCC1612
CAS No.:405911-09-3
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
- LXR-623
Catalog No.:BCC4273
CAS No.:875787-07-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104104-50-9 | SDF | Download SDF |
| PubChem ID | 107895 | Appearance | Powder |
| Formula | C15H18BrN3O3 | M.Wt | 368.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Gabazine | ||
| Solubility | DMSO : ≥ 75 mg/mL (203.68 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[6-imino-3-(4-methoxyphenyl)pyridazin-1-yl]butanoic acid;hydrobromide | ||
| SMILES | COC1=CC=C(C=C1)C2=NN(C(=N)C=C2)CCCC(=O)O.Br | ||
| Standard InChIKey | GFZHNFOGCMEYTA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H17N3O3.BrH/c1-21-12-6-4-11(5-7-12)13-8-9-14(16)18(17-13)10-2-3-15(19)20;/h4-9,16H,2-3,10H2,1H3,(H,19,20);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, competitive GABAA receptor antagonist. Displaces [3H]-GABA from rat brain membranes with a Ki of 150 nM. Unlike bicuculline, selectively antagonizes GABA-induced Cl- currents with little action on pentobarbitone-induced currents. Also acts as a low affinity glycine receptor antagonist. |

SR 95531 hydrobromide Dilution Calculator

SR 95531 hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7157 mL | 13.5785 mL | 27.1569 mL | 54.3139 mL | 67.8923 mL |
| 5 mM | 0.5431 mL | 2.7157 mL | 5.4314 mL | 10.8628 mL | 13.5785 mL |
| 10 mM | 0.2716 mL | 1.3578 mL | 2.7157 mL | 5.4314 mL | 6.7892 mL |
| 50 mM | 0.0543 mL | 0.2716 mL | 0.5431 mL | 1.0863 mL | 1.3578 mL |
| 100 mM | 0.0272 mL | 0.1358 mL | 0.2716 mL | 0.5431 mL | 0.6789 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-D-Glu(OtBu)-OH
Catalog No.:BCC3496
CAS No.:104091-08-9
- Zolantidine dimaleate
Catalog No.:BCC6922
CAS No.:104076-39-3
- Atipamezole hydrochloride
Catalog No.:BCC7521
CAS No.:104075-48-1
- Cyclo(Ile-Val)
Catalog No.:BCN2410
CAS No.:104068-43-1
- Rehmapicroside
Catalog No.:BCN2884
CAS No.:104056-82-8
- 3',5'-Diprenylgenistein
Catalog No.:BCN3572
CAS No.:104055-80-3
- Dihydroobovatin
Catalog No.:BCN3982
CAS No.:104055-79-0
- Quinovic acid 3-O-alpha-L-rhamnopyranoside
Catalog No.:BCN1636
CAS No.:104055-76-7
- LP533401 hcl
Catalog No.:BCC6377
CAS No.:1040526-12-2
- Trospium chloride
Catalog No.:BCC4582
CAS No.:10405-02-4
- 8alpha-Methacryloyloxybalchanin
Catalog No.:BCN4756
CAS No.:104021-39-8
- Tussilagone
Catalog No.:BCN2770
CAS No.:104012-37-5
- Kadsuric acid 3-methylester
Catalog No.:BCN3186
CAS No.:1041070-16-9
- Phellodendrine chloride
Catalog No.:BCN5934
CAS No.:104112-82-5
- Eldecalcitol
Catalog No.:BCC1548
CAS No.:104121-92-8
- Peramivir Trihydrate
Catalog No.:BCC4956
CAS No.:1041434-82-5
- Kuguaglycoside C
Catalog No.:BCN3276
CAS No.:1041631-93-9
- MitoPY1
Catalog No.:BCC6177
CAS No.:1041634-69-8
- Alpinoid D
Catalog No.:BCN3593
CAS No.:1041740-13-9
- Stanozolol
Catalog No.:BCC9154
CAS No.:10418-03-8
- 10-Nitro-camptothecin
Catalog No.:BCN2581
CAS No.:104195-61-1
- Estriol 3,17-dihexanoate
Catalog No.:BCN2238
CAS No.:104202-96-2
- Yunnandaphninine G
Catalog No.:BCN5857
CAS No.:1042143-83-8
- IRAK inhibitor 1
Catalog No.:BCC1654
CAS No.:1042224-63-4
Effects of endocannabinoids on discharge of baroreceptive NTS neurons.[Pubmed:15896495]
Neurosci Lett. 2005 Jun 24;381(3):334-9.
Previously, we have shown that microinjection of endocannabinoids (ECBs) into the nucleus tractus solitarius (NTS) can modulate baroreflex control of blood pressure (BP), prolonging pressor-induced inhibition of renal sympathetic nerve activity. This suggests that ECBs can modulate excitability of baroreceptive neurons in the NTS. Studies by others have shown that neural cannabinoid (CB1) receptors are present on fibers in the NTS, suggesting that some presynaptic modulation of transmitter release could occur in this region which receives direct afferent projections from arterial baroreceptors and cardiac mechanoreceptors. This study, therefore, was performed to determine the effects of ECBs on NTS baroreceptive neuronal discharge. Picoinjection of the ECB anandamide (AEA) was found to significantly increase discharge of baroreceptive neurons (20 of 23). Picoinjection of the ECB uptake inhibitor, AM404, which enhances endogenous ECB activity, also significantly increased discharge of baroreceptive neurons (8 of 10 neurons). To determine if effects of ECBs involved a GABAA mechanism, the neuronal responses to AEA and AM404 were tested after prior blockade of postsynaptic GABAA receptors by bicuculline (BIC) or SR 95531 hydrobromide (gabazine--SR 95531), which would eliminate any effects due to modulation of GABA input. The increase in neuronal discharge to both AEA and AM404 was significantly attenuated following BIC or SR 95531, which alone significantly increased discharge of baroreceptive neurons tested. These results support the hypothesis that ECBs enhance baroreflex function through increases in NTS baroreceptive neuronal activity, due in part to modulation of GABAergic inhibitory effects at the neuronal level.
The kinetics of inhibition of rat recombinant heteromeric alpha1beta glycine receptors by the low-affinity antagonist SR-95531.[Pubmed:17218350]
J Physiol. 2007 Apr 1;580(Pt 1):171-9.
The GABA(A) antagonist SR-95531 (gabazine) is known to block glycine receptors, albeit with low affinity. We have studied the effect of SR-95531 on rat recombinant alpha1beta glycine receptors expressed in human embryonic kidney (HEK293) cells by recording macroscopic currents elicited by rapid glycine application to outside-out patches. SR-95531 has a fast unbinding rate (k(offSR), about 3000 s(-1)) and this means that the time course of its unbinding is comparable to the expected time course of the transmitter in the cleft. We also found that equilibrium applications of SR-95531 reduced the response to brief glycine applications by an amount inversely proportional to the duration of glycine application. The fast unbinding rate of SR-95531 from the glycine receptor will make it useful for establishing the time course of glycine concentration at glycinergic synapses.
The differential antagonism by bicuculline and SR95531 of pentobarbitone-induced currents in cultured hippocampal neurons.[Pubmed:8831109]
Eur J Pharmacol. 1996 Jun 20;307(1):89-96.
In voltage clamped cultured hippocampal neurons, application of gamma-aminobutyric acid (GABA) or pentobarbitone induced chloride current in a dose-dependent manner. The dose dependence of these agonists were well described by ED50 and Hill coefficients of 14.7 +/- 7 microM and 1.2 +/- 0.5, and 299 +/- 17.3 microM and 1.6 +/- 0.1, for GABA and pentobarbitone, respectively. The effects of two GABAA receptor antagonists, bicuculline and 2-(3-carboxypropyl)-3-amino-6-methoxyphenyl-pyridazinium bromide (SR95531) were evaluated by co-application of increasing concentrations of the antagonists with a fixed equipotent (approximately ED30) dose of GABA or pentobarbitone. Both bicuculline and SR95531 blocked the GABA induced current with ID50 and Hill coefficients of 0.74 +/- 0.07 microM and 0.96 +/- 0.07, and 0.44 +/- 0.02 microM and 1.22 +/- 0.06, respectively. Bicuculline similarly blocked the pentobarbitone induced current with a ID50 and Hill coefficient of 0.69 +/- 0.04 microM and 1.2 +/- 0.1. However, pentobarbitone induced current was poorly blocked by SR95531 retaining 86.5% of current amplitude at a concentration of SR95531, 200 times the IC50 for GABA induced current. Current induced by etomidate, another intravenous general anesthetic with GABAA receptor agonistic property, is likewise resistant to SR95531 blockade. Three-dimensional modeling of bicuculline and SR95531 with alignment of the molecules along the suggested GABA-receptor binding moiety indicates that these two antagonist molecules have distinct steric properties. We suggest that GABA and pentobarbitone act at nearby but non-identical sites on the hippocampal GABAA receptor to open the chloride ionophore, and that these sites can be distinguished by bicuculline and SR95531. This is the first demonstration that the prototypic GABAA site antagonists bicuculline and SR95531 have different effects on currents induced by GABA and pentobarbitone.
Biochemical characterization of the interaction of three pyridazinyl-GABA derivatives with the GABAA receptor site.[Pubmed:3022866]
Brain Res. 1986 Oct 8;384(2):224-31.
An arylaminopyridazine derivative of gamma-aminobutyric acid (GABA), SR 95103, has been shown to be a selective antagonist of GABA at the GABAA receptor site. Subsequent structure-activity studies showed that suppressing the methyl in the 4-position of the pyridazine ring, and substituting the phenyl ring at the para position with a chlorine (SR 42641) or a methoxy group (SR 95531) led to compounds which exhibited the highest affinities for the GABA receptor site in this series. In the present study we examined the biochemical interaction of these compounds with the GABA receptor as well as their biochemical selectivity for this receptor. SR 95531 and SR 42641 displaced [3H]GABA from rat brain membranes with apparent Ki values of 0.15 microM and 0.28 microM respectively and Hill numbers near 1.0. The two compounds antagonized the GABA-elicited enhancement of [3H]diazepam-binding in a concentration-dependent manner without affecting [3H]diazepam-binding per se. Scatchard and Lineweaver-Burk analysis of the interaction of the two compounds with the GABAA receptor sites, revealed that the compounds were competitive at the high affinity site, but non-competitive at the low affinity site. Neither compound interacted with other GABAergic processes or with a variety of central receptor sites. When administered intravenously, SR 95531 and SR 42641 elicited tonic-clonic seizures in mice. Based on these results, it is postulated that SR 95531 and SR 42641 are specific, potent and competitive GABAA antagonists.


