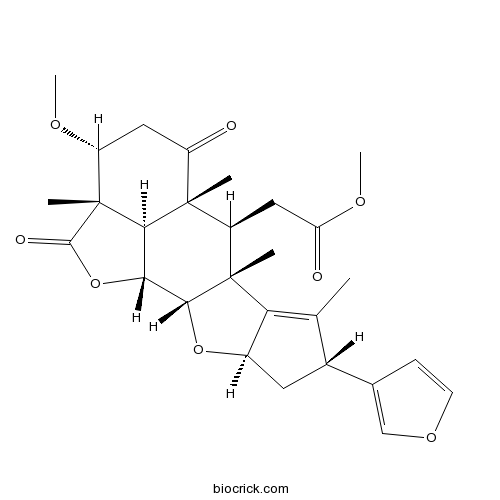
2,3-Dihydro-3alpha-methoxynimbolideCAS# 1607828-35-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1607828-35-2 | SDF | Download SDF |
| PubChem ID | 102200378 | Appearance | Powder |
| Formula | C28H34O8 | M.Wt | 498.57 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1=C2C(CC1C3=COC=C3)OC4C2(C(C5(C6C4OC(=O)C6(C(CC5=O)OC)C)C)CC(=O)OC)C | ||
| Standard InChIKey | OROAUBFPFAPQMD-LPLKPKLGSA-N | ||
| Standard InChI | InChI=1S/C28H34O8/c1-13-15(14-7-8-34-12-14)9-16-21(13)27(3)17(10-20(30)33-6)26(2)18(29)11-19(32-5)28(4)23(26)22(24(27)35-16)36-25(28)31/h7-8,12,15-17,19,22-24H,9-11H2,1-6H3/t15-,16-,17-,19-,22-,23-,24-,26+,27-,28-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 2,3-Dihydro-3α-methoxynimbolide induces apoptosis via both mitochondrial and death receptor pathways in stomach (AZ521) cancer cell line. |
| Targets | Bcl-2/Bax | Caspase |

2,3-Dihydro-3alpha-methoxynimbolide Dilution Calculator

2,3-Dihydro-3alpha-methoxynimbolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0057 mL | 10.0287 mL | 20.0574 mL | 40.1147 mL | 50.1434 mL |
| 5 mM | 0.4011 mL | 2.0057 mL | 4.0115 mL | 8.0229 mL | 10.0287 mL |
| 10 mM | 0.2006 mL | 1.0029 mL | 2.0057 mL | 4.0115 mL | 5.0143 mL |
| 50 mM | 0.0401 mL | 0.2006 mL | 0.4011 mL | 0.8023 mL | 1.0029 mL |
| 100 mM | 0.0201 mL | 0.1003 mL | 0.2006 mL | 0.4011 mL | 0.5014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,4,6-Trihydroxy-5-methoxy-7-prenylxanthone
Catalog No.:BCN1548
CAS No.:160623-47-2
- Villosin
Catalog No.:BCN1711
CAS No.:160598-92-5
- DMP 543
Catalog No.:BCC7331
CAS No.:160588-45-4
- Zarzissine
Catalog No.:BCN6456
CAS No.:160568-14-9
- 5,7,3',4'-Tetrahydroxy-3-methoxy-8-geranylflavone
Catalog No.:BCN6847
CAS No.:1605304-56-0
- BW 723C86 hydrochloride
Catalog No.:BCC6915
CAS No.:160521-72-2
- 12S-hydroxyandrographolide
Catalog No.:BCN4700
CAS No.:869593-50-0
- Bisandrographolide A
Catalog No.:BCN4701
CAS No.:160498-00-0
- Antirhine
Catalog No.:BCN4003
CAS No.:16049-28-8
- THZ2
Catalog No.:BCC3986
CAS No.:1604810-84-5
- THZ1
Catalog No.:BCC4005
CAS No.:1604810-83-4
- 30-Oxopseudotaraxasterol
Catalog No.:BCN7135
CAS No.:160481-71-0
- Ardisicrenoside B
Catalog No.:BCN8078
CAS No.:160791-12-8
- Indirubin-3'-oxime
Catalog No.:BCC7185
CAS No.:160807-49-8
- AMG319
Catalog No.:BCC6510
CAS No.:1608125-21-8
- H-Thr(Bzl)-ol
Catalog No.:BCC2577
CAS No.:160841-03-2
- Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate
Catalog No.:BCC8967
CAS No.:160844-75-7
- Chrysin 8-C-glucoside
Catalog No.:BCN7982
CAS No.:160880-89-7
- Fmoc-Valinol
Catalog No.:BCC2694
CAS No.:160885-98-3
- Hosenkoside F
Catalog No.:BCN2520
CAS No.:160896-45-7
- Hosenkoside G
Catalog No.:BCN2272
CAS No.:160896-46-8
- Hosenkoside K
Catalog No.:BCN2577
CAS No.:160896-49-1
- Villosin C
Catalog No.:BCN1712
CAS No.:160927-81-1
- AZD3264
Catalog No.:BCC6514
CAS No.:1609281-86-8
Limonoids from Azadirachta indica var. siamensis extracts and their cytotoxic and melanogenesis-inhibitory activities.[Pubmed:24706622]
Chem Biodivers. 2014 Apr;11(4):505-31.
Six new limonoids, 7-benzoyl-17-epinimbocinol (5), 3-acetyl-7-tigloylnimbidinin (8), 1-isovaleroyl-1-detigloylsalanninolide (15), 2,3-Dihydro-3alpha-methoxynimbolide (16), deacetyl-20,21-epoxy-20,22-dihydro-21-deoxyisonimbinolide (26), and deacetyl-20,21,22,23-tetrahydro-20,22-dihydroxy-21,23-dimethoxynimbin (27), along with 28 known limonoids, 1-4, 6, 7, 9-14, 17-25, and 28-34, and two known flavonoids, 35 and 36, have been isolated from the extracts of bark, leaves, roots, and seeds of Azadirachta indica A. Juss. var. siamensis Valeton (Siamese neem tree; Meliaceae). The structures of the new compounds were elucidated on the basis of extensive spectroscopic analysis and comparison with literature data. All of these compounds were evaluated for their cytotoxic activities against leukemia (HL60), lung (A549), stomach (AZ521), and breast (SK-BR-3) cancer cell lines. Eleven compounds, 1, 2, 4-7, 13, 16, 17, 29, and 30, exhibited potent cytotoxicities against one or more cell lines with IC50 values in the range of 0.1-9.3 muM. Compound 16 induced apoptotic cell death in AZ521 cells upon evaluation of the apoptosis-inducing activity by flow cytometric analysis. Western blot analysis on AZ521 cells revealed that compound 16 activated caspases-3, -8, and -9, while increasing the ratio of Bax/Bcl-2. This suggested that 16 induced apoptosis via both mitochondrial and death receptor pathways in AZ521. In addition, upon evaluation of all compounds against the melanogenesis in B16 melanoma cells induced with alpha-melanocyte-stimulating hormone (alpha-MSH), 20 limonoids, i.e., 1-3, 6, 9-11, 18, 19, 21-29, 32, and 34, and two flavonoids, 35 and 36, exhibited melanogenesis-inhibitory activities, with no, or almost no, toxicities to the cells at lower and/or higher concentrations, which were more potent than the reference arbutin, a known melanogenesis inhibitor. Western blot analysis showed that nimbin (18) reduced the protein levels of microphtalmia-associated transcription factor (MITF), tyrosinase, tyrosine-related protein 1 (TRP-1), and TRP-2 mostly in a concentration-dependent manner, indicating that 18 inhibits melanogenesis on a alpha-MSH-stimulated B16 melanoma cells by, at least in part, inhibiting the expression of MITF, followed by decreasing the expression of tyrosinase, TRP-1, and TRP-2.


