Ardisicrenoside BCAS# 160791-12-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 160791-12-8 | SDF | Download SDF |
| PubChem ID | 10373894 | Appearance | Powder |
| Formula | C52H86O22 | M.Wt | 1063.23 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
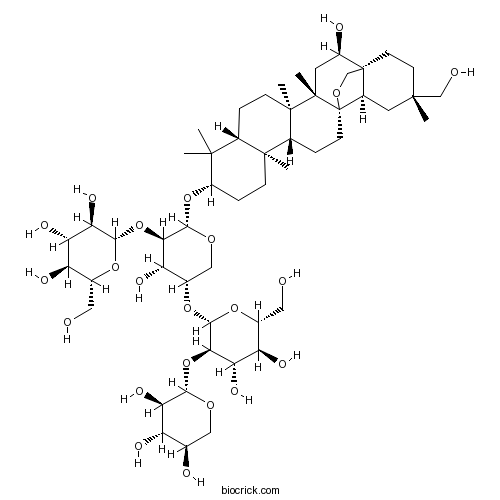
| Chemical Name | (2S,3R,4S,5S,6R)-2-[(2S,3R,4S,5S)-5-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxyoxan-2-yl]oxy-4-hydroxy-2-[[(1S,2R,4S,5R,8R,10S,13R,14R,17S,18R,20S)-2-hydroxy-20-(hydroxymethyl)-4,5,9,9,13,20-hexamethyl-24-oxahexacyclo[15.5.2.01,18.04,17.05,14.08,13]tetracosan-10-yl]oxy]oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC1(C2CCC3(C(C2(CCC1OC4C(C(C(CO4)OC5C(C(C(C(O5)CO)O)O)OC6C(C(C(CO6)O)O)O)O)OC7C(C(C(C(O7)CO)O)O)O)C)CCC89C3(CC(C1(C8CC(CC1)(C)CO)CO9)O)C)C)C | ||
| Standard InChIKey | RDBUKNALARTFFW-VKKSEJOZSA-N | ||
| Standard InChI | InChI=1S/C52H86O22/c1-46(2)27-7-11-49(5)28(8-12-52-29-15-47(3,21-55)13-14-51(29,22-68-52)30(57)16-50(49,52)6)48(27,4)10-9-31(46)72-44-40(74-43-39(65)36(62)33(59)24(17-53)69-43)35(61)26(20-67-44)71-45-41(37(63)34(60)25(18-54)70-45)73-42-38(64)32(58)23(56)19-66-42/h23-45,53-65H,7-22H2,1-6H3/t23-,24-,25-,26+,27+,28-,29-,30-,31+,32+,33-,34-,35+,36+,37+,38-,39-,40-,41-,42+,43+,44+,45+,47+,48+,49-,50+,51-,52+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Quantitative determination of triperpene saponins and alkenated-phenolics from Labisia pumila using an LC-UV/ELSD method and confirmation by LC-ESI-TOF.[Pubmed: 21590653 ]Planta Med. 2011 Oct;77(15):1742-8.This study describes the first analytical method for the determination of four triterpene saponins (Ardisicrenoside B, ardisiacrispin A, 3- O- α- L-rhamnopyranosyl-(1 → 2)-β-D-glucopyranosyl-(1 → 4)-α-L-arabinopynanosyl cyclamiretin A and ardisimamilloside H) and three alkenated-phenolics (irisresorcinol, belamcandol B, and demethylbelamcandaquinone B) from the leaves, leaves/stems, and roots of LABISIA PUMILA using an HPLC-UV-ELSD method.
|
| Structure Identification | J Asian Nat Prod Res. 2008 Mar-Apr;10(3-4):291-6.Triterpene saponins from Lysimachia christinae.[Pubmed: 18335347 ]
|

Ardisicrenoside B Dilution Calculator

Ardisicrenoside B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.9405 mL | 4.7027 mL | 9.4053 mL | 18.8106 mL | 23.5133 mL |
| 5 mM | 0.1881 mL | 0.9405 mL | 1.8811 mL | 3.7621 mL | 4.7027 mL |
| 10 mM | 0.0941 mL | 0.4703 mL | 0.9405 mL | 1.8811 mL | 2.3513 mL |
| 50 mM | 0.0188 mL | 0.0941 mL | 0.1881 mL | 0.3762 mL | 0.4703 mL |
| 100 mM | 0.0094 mL | 0.047 mL | 0.0941 mL | 0.1881 mL | 0.2351 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,3-Dihydro-3alpha-methoxynimbolide
Catalog No.:BCN7093
CAS No.:1607828-35-2
- 1,4,6-Trihydroxy-5-methoxy-7-prenylxanthone
Catalog No.:BCN1548
CAS No.:160623-47-2
- Villosin
Catalog No.:BCN1711
CAS No.:160598-92-5
- DMP 543
Catalog No.:BCC7331
CAS No.:160588-45-4
- Zarzissine
Catalog No.:BCN6456
CAS No.:160568-14-9
- 5,7,3',4'-Tetrahydroxy-3-methoxy-8-geranylflavone
Catalog No.:BCN6847
CAS No.:1605304-56-0
- BW 723C86 hydrochloride
Catalog No.:BCC6915
CAS No.:160521-72-2
- 12S-hydroxyandrographolide
Catalog No.:BCN4700
CAS No.:869593-50-0
- Bisandrographolide A
Catalog No.:BCN4701
CAS No.:160498-00-0
- Antirhine
Catalog No.:BCN4003
CAS No.:16049-28-8
- THZ2
Catalog No.:BCC3986
CAS No.:1604810-84-5
- THZ1
Catalog No.:BCC4005
CAS No.:1604810-83-4
- Indirubin-3'-oxime
Catalog No.:BCC7185
CAS No.:160807-49-8
- AMG319
Catalog No.:BCC6510
CAS No.:1608125-21-8
- H-Thr(Bzl)-ol
Catalog No.:BCC2577
CAS No.:160841-03-2
- Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate
Catalog No.:BCC8967
CAS No.:160844-75-7
- Chrysin 8-C-glucoside
Catalog No.:BCN7982
CAS No.:160880-89-7
- Fmoc-Valinol
Catalog No.:BCC2694
CAS No.:160885-98-3
- Hosenkoside F
Catalog No.:BCN2520
CAS No.:160896-45-7
- Hosenkoside G
Catalog No.:BCN2272
CAS No.:160896-46-8
- Hosenkoside K
Catalog No.:BCN2577
CAS No.:160896-49-1
- Villosin C
Catalog No.:BCN1712
CAS No.:160927-81-1
- AZD3264
Catalog No.:BCC6514
CAS No.:1609281-86-8
- Mps1-IN-3
Catalog No.:BCC5496
CAS No.:1609584-72-6
Quantitative determination of triperpene saponins and alkenated-phenolics from Labisia pumila using an LC-UV/ELSD method and confirmation by LC-ESI-TOF.[Pubmed:21590653]
Planta Med. 2011 Oct;77(15):1742-8.
This study describes the first analytical method for the determination of four triterpene saponins (Ardisicrenoside B, ardisiacrispin A, 3- O- alpha- L-rhamnopyranosyl-(1 --> 2)-beta-D-glucopyranosyl-(1 --> 4)-alpha-L-arabinopynanosyl cyclamiretin A and ardisimamilloside H) and three alkenated-phenolics (irisresorcinol, belamcandol B, and demethylbelamcandaquinone B) from the leaves, leaves/stems, and roots of LABISIA PUMILA using an HPLC-UV-ELSD method. The separation was achieved using a reversed-phase (C-18) column, PDA and ELS detection, and a water/acetonitrile gradient as the mobile phase. The major triterpenoid (ardisiacrispin A) and irisresorcinol compounds were detected at a concentration as low as 10.0 and 0.2 microg/mL, respectively. Analysis of various samples showed considerable variation of 0.11-2.46 % for the major triterpenoid compound, ardisiacrispin A. LC-mass spectrometry method coupled with electrospray ionization (ESI) is described for the identification of compounds in plant samples. This method involved the use of the [M + Na]+ and [M + NH(4)]+ ions for compounds 1-4 in the positive ion mode with extractive ion chromatogram (EIC).
Triterpene saponins from Lysimachia christinae.[Pubmed:18335347]
J Asian Nat Prod Res. 2008 Mar-Apr;10(3-4):291-6.
Seven triterpene saponins were isolated from Lysimachia christinae and identified as lysichriside A (1), lysichriside B (2), primulanin (3), lysikokianoside 1 (4), anagallisin C (5), ardisiacrispin A (6), and Ardisicrenoside B (7). Compounds 1 and 2 are new triterpene saponins, compounds 3, 5, and 7 were isolated from this genus, and compounds 4 and 6 were isolated from this species for the first time. Their structures were elucidated by means of 1D and 2D NMR experiments.


