2-AcetylbutyrolactoneCAS# 517-23-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 517-23-7 | SDF | Download SDF |
| PubChem ID | 10601 | Appearance | Powder |
| Formula | C6H8O3 | M.Wt | 128 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-acetyloxolan-2-one | ||
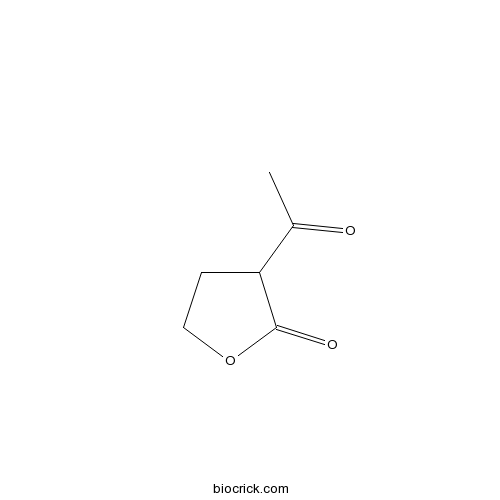
| SMILES | CC(=O)C1CCOC1=O | ||
| Standard InChIKey | OMQHDIHZSDEIFH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H8O3/c1-4(7)5-2-3-9-6(5)8/h5H,2-3H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Acetylbutyrolactone Dilution Calculator

2-Acetylbutyrolactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.8125 mL | 39.0625 mL | 78.125 mL | 156.25 mL | 195.3125 mL |
| 5 mM | 1.5625 mL | 7.8125 mL | 15.625 mL | 31.25 mL | 39.0625 mL |
| 10 mM | 0.7813 mL | 3.9063 mL | 7.8125 mL | 15.625 mL | 19.5313 mL |
| 50 mM | 0.1563 mL | 0.7813 mL | 1.5625 mL | 3.125 mL | 3.9063 mL |
| 100 mM | 0.0781 mL | 0.3906 mL | 0.7813 mL | 1.5625 mL | 1.9531 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kadsurin
Catalog No.:BCN3634
CAS No.:51670-40-7
- Erythrartine
Catalog No.:BCN5642
CAS No.:51666-26-3
- 2-Methoxyphenalen-1-one
Catalog No.:BCN7181
CAS No.:51652-39-2
- Murrangatin diacetate
Catalog No.:BCN5641
CAS No.:51650-59-0
- BML-277
Catalog No.:BCC4245
CAS No.:516480-79-8
- Z-D-Glu(OtBu)-OH
Catalog No.:BCC2771
CAS No.:51644-83-8
- 20(S)-Hydroxycholesterol
Catalog No.:BCC7937
CAS No.:516-72-3
- Allopregnanolone
Catalog No.:BCC7737
CAS No.:516-54-1
- Cerevisterol
Catalog No.:BCN5640
CAS No.:516-37-0
- Taurochenodeoxycholic Acid
Catalog No.:BCN8419
CAS No.:516-35-8
- Methylmalonate
Catalog No.:BCC7986
CAS No.:516-05-2
- Cuspidiol
Catalog No.:BCN3942
CAS No.:51593-96-5
- Hematoxylin
Catalog No.:BCC5322
CAS No.:517-28-2
- Sennidin B
Catalog No.:BCN6355
CAS No.:517-44-2
- Corytuberine
Catalog No.:BCN2670
CAS No.:517-56-6
- Stephanine
Catalog No.:BCN5643
CAS No.:517-63-5
- Dicentrine
Catalog No.:BCN3296
CAS No.:517-66-8
- Shikonin
Catalog No.:BCN1006
CAS No.:517-88-4
- Shikonine
Catalog No.:BCN3530
CAS No.:517-89-5
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Estra-4,9-diene-3,17-dione
Catalog No.:BCC8959
CAS No.:5173-46-6
- Valechlorine
Catalog No.:BCN2763
CAS No.:51771-49-4
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
- Carteolol HCl
Catalog No.:BCC6466
CAS No.:51781-21-6
Application of 2-acetylbutyrolactone to spectrofluorimetry: fluorescence properties of Schiff bases derived from 2-acetylbutyrolactone and spectrofluorimetric determination of primary amine-containing compounds.[Pubmed:16256289]
J Pharm Biomed Anal. 2006 Mar 18;40(5):1057-67.
2-Acetylbutyrolactone (ABL) has been characterized for use as a fluorogenic reagent for the spectrofluorimetric determination of primary amines. The reagent forms strongly fluorescent Schiff bases upon the reaction with primary amines in acid-catalized aqueous solutions or in dimethylformamide (DMF). Sulfamethoxazole (SMX) and ampicillin sodium (AMP Na) were used as model amines of type ArNH(2) and RNH(2), respectively. The reaction conditions, fluorescence spectral properties and the stability of the derivatives have been investigated. The chemistry and the pathway of the reaction have been discussed. Calibration data, accuracy, precision, limits of detection, limits of quantification and other aspects of analytical merit were presented in the text. The utility of ABL for the analysis of the model drugs in pharmaceutical preparations was demonstrated. The results indicated that the proposed methods are equally accurate and precise as the official or other reported methods.


