CorytuberineCAS# 517-56-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 517-56-6 | SDF | Download SDF |
| PubChem ID | 160500 | Appearance | Powder |
| Formula | C19H21NO4 | M.Wt | 327.37 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
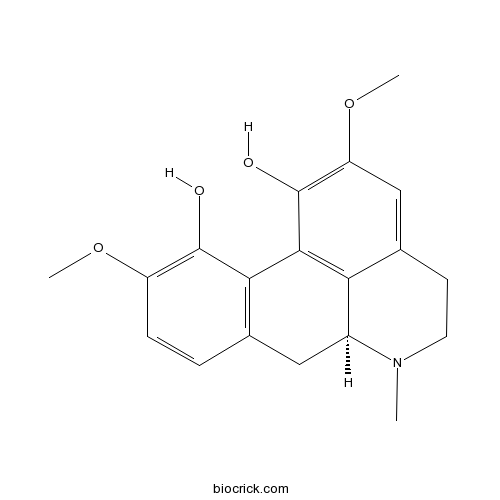
| Chemical Name | (6aS)-2,10-dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-1,11-diol | ||
| SMILES | CN1CCC2=CC(=C(C3=C2C1CC4=C3C(=C(C=C4)OC)O)O)OC | ||
| Standard InChIKey | WHFUDAOCYRYAKQ-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C19H21NO4/c1-20-7-6-11-9-14(24-3)19(22)17-15(11)12(20)8-10-4-5-13(23-2)18(21)16(10)17/h4-5,9,12,21-22H,6-8H2,1-3H3/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Corytuberine was inhibited only by naloxone and that of bulbocapnine preferentially by yohimbine. |

Corytuberine Dilution Calculator

Corytuberine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0546 mL | 15.2732 mL | 30.5465 mL | 61.093 mL | 76.3662 mL |
| 5 mM | 0.6109 mL | 3.0546 mL | 6.1093 mL | 12.2186 mL | 15.2732 mL |
| 10 mM | 0.3055 mL | 1.5273 mL | 3.0546 mL | 6.1093 mL | 7.6366 mL |
| 50 mM | 0.0611 mL | 0.3055 mL | 0.6109 mL | 1.2219 mL | 1.5273 mL |
| 100 mM | 0.0305 mL | 0.1527 mL | 0.3055 mL | 0.6109 mL | 0.7637 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sennidin B
Catalog No.:BCN6355
CAS No.:517-44-2
- Hematoxylin
Catalog No.:BCC5322
CAS No.:517-28-2
- 2-Acetylbutyrolactone
Catalog No.:BCC8515
CAS No.:517-23-7
- Kadsurin
Catalog No.:BCN3634
CAS No.:51670-40-7
- Erythrartine
Catalog No.:BCN5642
CAS No.:51666-26-3
- 2-Methoxyphenalen-1-one
Catalog No.:BCN7181
CAS No.:51652-39-2
- Murrangatin diacetate
Catalog No.:BCN5641
CAS No.:51650-59-0
- BML-277
Catalog No.:BCC4245
CAS No.:516480-79-8
- Z-D-Glu(OtBu)-OH
Catalog No.:BCC2771
CAS No.:51644-83-8
- 20(S)-Hydroxycholesterol
Catalog No.:BCC7937
CAS No.:516-72-3
- Allopregnanolone
Catalog No.:BCC7737
CAS No.:516-54-1
- Cerevisterol
Catalog No.:BCN5640
CAS No.:516-37-0
- Stephanine
Catalog No.:BCN5643
CAS No.:517-63-5
- Dicentrine
Catalog No.:BCN3296
CAS No.:517-66-8
- Shikonin
Catalog No.:BCN1006
CAS No.:517-88-4
- Shikonine
Catalog No.:BCN3530
CAS No.:517-89-5
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Estra-4,9-diene-3,17-dione
Catalog No.:BCC8959
CAS No.:5173-46-6
- Valechlorine
Catalog No.:BCN2763
CAS No.:51771-49-4
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
- Carteolol HCl
Catalog No.:BCC6466
CAS No.:51781-21-6
- Rengynic acid
Catalog No.:BCN5644
CAS No.:517883-38-4
- Dehydroglyasperin D
Catalog No.:BCN6829
CAS No.:517885-72-2
- Evodiamine
Catalog No.:BCN1092
CAS No.:518-17-2
Neuroleptic-like, anticonvulsant and antinociceptive effects of aporphine alkaloids: bulbocapnine, corytuberine, boldine and glaucine.[Pubmed:2907279]
Arch Int Pharmacodyn Ther. 1988 Nov-Dec;296:255-81.
The aporphine alkaloids bulbocapnine, Corytuberine, boldine and glaucine were studied in mice and compared with haloperidol, phenobarbital and morphine. All aporphines inhibited the exploratory rearing activity and elicited palpebral ptosis, catalepsy, hypothermia, and prolonged anesthesia by thiopental. They also reduced nociception (hot plate; phenylquinone-induced writhing) and (except for Corytuberine) were anticonvulsant against harman and picrotoxin, but not against bicuculline and pentetrazol; Corytuberine was proconvulsant. The aporphines (except for Corytuberine) antagonized the apomorphine- and methylphenidate-induced stereotyped gnawing and also the apomorphine-induced climbing activity; Corytuberine was prostereotypic. The antignawing effects (including those of haloperidol) were stronger when the antagonists were administered after the agonists (gnawing full-fletched) rather than before them: this led to the speculation of a metaphilic interaction at central site(s). Clonazepam inhibited the antistereotypic effect (vs apomorphine) more potently with the aporphines than with haloperidol. The antinociceptive effect (writhing) of the aporphines was, in contrast to that of morphine, resistant to both naloxone and yohimbine. The latter applied also to the antilicking action in the hot plate test; the antijumping effect of boldine was (like that of morphine) antagonized by both yohimbine and naloxone, whereas that of Corytuberine was inhibited only by naloxone and that of bulbocapnine preferentially by yohimbine. Hence, opioid and adrenergic mechanisms are unequally involved in the antinociceptive effects of the aporphines. The present results also showed that licking and jumping (in the hot plate test) are pharmacologically different phenomena. In low doses, the aporphines and haloperidol antagonized the antinociceptive effect of morphine (hot plate); hence, these drugs may be considered partial agonists or partial antagonists, respectively.


