DicentrineCAS# 517-66-8 |
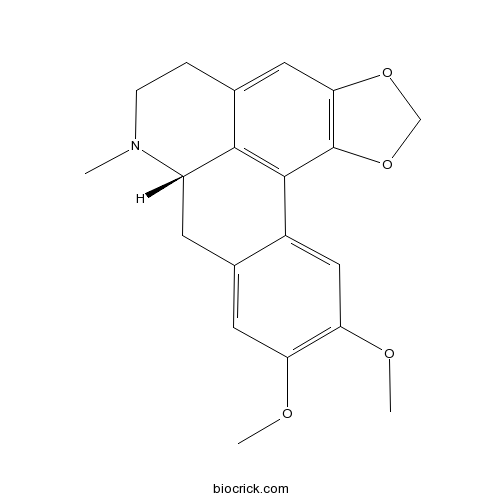
- (-)-dicentrine
Catalog No.:BCC8167
CAS No.:28832-07-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 517-66-8 | SDF | Download SDF |
| PubChem ID | 101300 | Appearance | Cryst. |
| Formula | C20H21NO4 | M.Wt | 339.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CN1CCC2=CC3=C(C4=C2C1CC5=CC(=C(C=C54)OC)OC)OCO3 | ||
| Standard InChIKey | YJWBWQWUHVXPNC-AWEZNQCLSA-N | ||
| Standard InChI | InChI=1S/C20H21NO4/c1-21-5-4-11-7-17-20(25-10-24-17)19-13-9-16(23-3)15(22-2)8-12(13)6-14(21)18(11)19/h7-9,14H,4-6,10H2,1-3H3/t14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dicentrine has antinociception in different models of chemical pain. 2. Dicentrine induces DNA lesions to arrest cell cycle. 3. Dicentrine, a selective α(1)-adrenoceptor antagonist with potent antiarrhythmic and antihypertensive activities. 4. Callus and cell suspension cultures of S. venosa can produce high levels of Dicentrine as an alternative source of plant materials. |

Dicentrine Dilution Calculator

Dicentrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9464 mL | 14.7319 mL | 29.4638 mL | 58.9275 mL | 73.6594 mL |
| 5 mM | 0.5893 mL | 2.9464 mL | 5.8928 mL | 11.7855 mL | 14.7319 mL |
| 10 mM | 0.2946 mL | 1.4732 mL | 2.9464 mL | 5.8928 mL | 7.3659 mL |
| 50 mM | 0.0589 mL | 0.2946 mL | 0.5893 mL | 1.1786 mL | 1.4732 mL |
| 100 mM | 0.0295 mL | 0.1473 mL | 0.2946 mL | 0.5893 mL | 0.7366 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Stephanine
Catalog No.:BCN5643
CAS No.:517-63-5
- Corytuberine
Catalog No.:BCN2670
CAS No.:517-56-6
- Sennidin B
Catalog No.:BCN6355
CAS No.:517-44-2
- Hematoxylin
Catalog No.:BCC5322
CAS No.:517-28-2
- 2-Acetylbutyrolactone
Catalog No.:BCC8515
CAS No.:517-23-7
- Kadsurin
Catalog No.:BCN3634
CAS No.:51670-40-7
- Erythrartine
Catalog No.:BCN5642
CAS No.:51666-26-3
- 2-Methoxyphenalen-1-one
Catalog No.:BCN7181
CAS No.:51652-39-2
- Murrangatin diacetate
Catalog No.:BCN5641
CAS No.:51650-59-0
- BML-277
Catalog No.:BCC4245
CAS No.:516480-79-8
- Z-D-Glu(OtBu)-OH
Catalog No.:BCC2771
CAS No.:51644-83-8
- 20(S)-Hydroxycholesterol
Catalog No.:BCC7937
CAS No.:516-72-3
- Shikonin
Catalog No.:BCN1006
CAS No.:517-88-4
- Shikonine
Catalog No.:BCN3530
CAS No.:517-89-5
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Estra-4,9-diene-3,17-dione
Catalog No.:BCC8959
CAS No.:5173-46-6
- Valechlorine
Catalog No.:BCN2763
CAS No.:51771-49-4
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
- Carteolol HCl
Catalog No.:BCC6466
CAS No.:51781-21-6
- Rengynic acid
Catalog No.:BCN5644
CAS No.:517883-38-4
- Dehydroglyasperin D
Catalog No.:BCN6829
CAS No.:517885-72-2
- Evodiamine
Catalog No.:BCN1092
CAS No.:518-17-2
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- (-)-beta-Peltatin
Catalog No.:BCN3606
CAS No.:518-29-6
Dicentrine production in callus and cell suspension cultures of Stephania venosa.[Pubmed:23738448]
Nat Prod Commun. 2013 Apr;8(4):443-5.
The highest Dicentrine content (19.5 +/- 0.3 mg/g dry weight) from callus culture of Stephania venosa was achieved from stem segments cultured on MS medium supplemented with TDZ 0.5 mg/L and NAA 1.0 mg/L. Cell suspension cultures were established from callus cultured on MS liquid medium with the same plant growth regulators. Dicentrine production from S. venosa cell suspension cultures was obtained in the range of 15-26 mg/g dry weight. Elicitation in cell suspension cultures by chitosan (50 mg/L) and salicylic acid (2 mg/L) for 6 days significantly increased Dicentrine content. Our findings indicate that callus and cell suspension cultures of S. venosa can produce high levels of Dicentrine as an alternative source of plant materials.
Antinociceptive effects of a chloroform extract and the alkaloid dicentrine isolated from fruits of Ocotea puberula.[Pubmed:22815198]
Planta Med. 2012 Sep;78(14):1543-8.
The present work describes the chemical characterization of a chloroform fraction (CF) obtained from an extract of Ocotea puberula (Lauraceae) fruits, and preliminary antinociceptive analysis of CF and the alkaloid Dicentrine, isolated from this fraction. CF (30-300 mg/kg, p. o.) caused dose-related inhibition of abdominal constrictions caused by acetic acid and also inhibited both phases of formalin-induced nociception. However, hexane or ethyl acetate fractions did not produce any effect. Antinociception caused by CF (100 mg/kg, p. o.) in the acetic acid test was not affected either by caffeine, an adenosine receptor antagonist, or by naloxone, an opioid receptor antagonist, and neither was associated with nonspecific effects such as muscle relaxation or sedation. Furthermore, Dicentrine (30-300 mg/kg, p. o.) produced dose-related inhibition of acetic acid-induced pain without causing changes in the motor performance of mice. The results show, for the first time, that CF from Ocotea puberula fruits produced marked antinociception in different models of chemical pain, and this effect appears to be, at least in part, due to the presence of Dicentrine. The mechanism by which CF and the alkaloid produced antinociception still remains unclear, but the adenosinergic or opioid system seems unlikely to be involved in this action.
Metabolism of dicentrine: identification of the phase I and phase II metabolites in miniature pig urine.[Pubmed:20622045]
Drug Metab Dispos. 2010 Oct;38(10):1714-22.
The metabolic profile of Dicentrine, a selective alpha(1)-adrenoceptor antagonist with potent antiarrhythmic and antihypertensive activities, in miniature pig urine via oral administration was investigated for the first time. The urine, collected after a single oral administration of Dicentrine, was pretreated using solvent extraction and column chromatographic methods to identify the metabolites containing fractions. Twenty-four metabolites (MI-1-9 and MII-1-15), of which 21 compounds are new, were identified by mass spectrometry and high-performance liquid chromatography-diode array detector solid-phase extraction-NMR techniques. Of these, 14 metabolites (MI-5, MII-1 and 2, and MII-5-15) were further isolated for structure confirmation. The phase I metabolic transformations of Dicentrine were found to be N-demethylation, N-oxidation, O-demethylation (9,10-OMe), O,O-demethylenation (1-OCH(2)O-2), and hydroxylation at the benzylic (C-4) and the aromatic (C-3) positions, whereas those for the phase II were O-glucuronidation and O-glucosylation of the phenolic group of the phase I metabolites.
Inhibition of epidermal growth factor receptor over-expressing cancer cells by the aphorphine-type isoquinoline alkaloid, dicentrine.[Pubmed:20005213]
Biochem Pharmacol. 2010 Apr 15;79(8):1092-9.
The extraordinary relevance of EGFR in tumour biology makes it an exquisite molecular target for tumour therapy. Despite considerable success with these EGFR tyrosine kinase inhibitors in cancer therapy, resistance against these chemical compounds develops owing to the selection of point-mutated variants of EGFR. Therefore, there is an urgent need for the identification of novel EGFR tyrosine kinase inhibitors for treating tumours with such EGFR mutants. We found a preferential cytotoxicity of Dicentrine towards U87MG.DeltaEGFR-transduced with a constitutively deletion-activated EGFR expression vector as compared to non-transduced wild-type U87MG cells. As determined by microarray-based mRNA expression profiling, this preferential cytotoxicity was accompanied with an activation of BRCA1-mediated DNA damage response, p53 signalling, G1/S and G2/M cell cycle regulation, and aryl hydrocarbon receptor pathways. The activation of these signalling routes might be explained by the fact that Dicentrine intercalates DNA and induces DNA strand break by inhibition of DNA topoisomerases. The cell cycle might be arrested by Dicentrine-induced DNA lesions.


