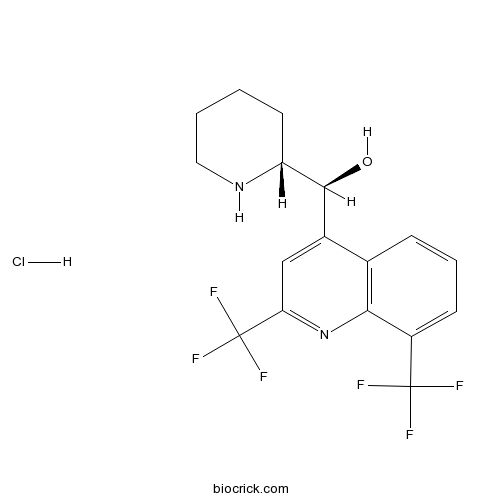
Mefloquine hydrochlorideQuinoline methanol antimalarial agent, CAS# 51773-92-3 |
- Salmefamol
Catalog No.:BCC1919
CAS No.:18910-65-1
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51773-92-3 | SDF | Download SDF |
| PubChem ID | 65329 | Appearance | Powder |
| Formula | C17H17ClF6N2O | M.Wt | 414.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Mefloquin hydrochloride | ||
| Solubility | DMSO : ≥ 100 mg/mL (241.10 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [2,8-bis(trifluoromethyl)quinolin-4-yl]-(2-piperidyl)methanol | ||
| SMILES | [H+].[Cl-].OC(C1CCCCN1)c2cc(nc3c2cccc3C(F)(F)F)C(F)(F)F | ||
| Standard InChIKey | WESWYMRNZNDGBX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H16F6N2O.ClH/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11;/h3-5,8,12,15,24,26H,1-2,6-7H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mefloquine hydrochloride is a quinoline antimalarial drug that is structurally related to the antiarrhythmic agent quinidine.
IC50 Value: 1 microM ( for K+ channel) [1]
Target: Antiparasitic
Mefloquine is widely used in both the treatment and prophylaxis of Plasmodium falciparum malaria. MQ can induces oxidative stress in vitro. Evidence indicates that reactive oxygen species (ROS) may be used as a therapeutic modality to kill cancer cells [2].
in vitro: Mefloquine inhibitedKvLQT1/minK channel currents with an IC50 value of approximately 1 microM. Mefloquine slowed the activation rate of KvLQT1/minK and more block was evident at lower membrane potentials compared with higher ones. HERG channel currents were about 6-fold less sensitive to block by mefloquine (IC50 = 5.6 microM). Block of HERG displayed a positive voltage dependence with maximal inhibition obtained at more depolarized potentials [1]. MQ has a highly selective cytotoxicity that inhibits PCa cell growth. MQ-mediated ROS simultaneously downregulated Akt phosphorylation and activated extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and adenosine monophosphate-activated protein kinase (AMPK) signaling in PC3 cells [2].
in vivo: Pregnant rats were treated orally with AS (15 and 40 mg/kg body weight (bwt)/day), MQ (30 and 80 mg/kg bwt/day) and AS/MQ (15/30 and 40/80 mg/kg bwt/day) on days 9-11 post coitum (pc). The dams were euthanized on day 12 pc and gestational and embryos histological parameters were evaluated [3].
Clinical trial: Activity of Mefloquine Against Urinary Schistosomiasis . Phase 2 References: | |||||

Mefloquine hydrochloride Dilution Calculator

Mefloquine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.411 mL | 12.0549 mL | 24.1097 mL | 48.2195 mL | 60.2744 mL |
| 5 mM | 0.4822 mL | 2.411 mL | 4.8219 mL | 9.6439 mL | 12.0549 mL |
| 10 mM | 0.2411 mL | 1.2055 mL | 2.411 mL | 4.8219 mL | 6.0274 mL |
| 50 mM | 0.0482 mL | 0.2411 mL | 0.4822 mL | 0.9644 mL | 1.2055 mL |
| 100 mM | 0.0241 mL | 0.1205 mL | 0.2411 mL | 0.4822 mL | 0.6027 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 1 microM ( for K+ channel) [1] Mefloquine is a quinoline antimalarial drug that is structurally related to the antiarrhythmic agent quinidine. Mefloquine is widely used in both the treatment and prophylaxis of Plasmodium falciparum malaria. MQ can induces oxidative stress in vitro. Evidence indicates that reactive oxygen species (ROS) may be used as a therapeutic modality to kill cancer cells [2]. in vitro: Mefloquine inhibitedKvLQT1/minK channel currents with an IC50 value of approximately 1 microM. Mefloquine slowed the activation rate of KvLQT1/minK and more block was evident at lower membrane potentials compared with higher ones. HERG channel currents were about 6-fold less sensitive to block by mefloquine (IC50 = 5.6 microM). Block of HERG displayed a positive voltage dependence with maximal inhibition obtained at more depolarized potentials [1]. MQ has a highly selective cytotoxicity that inhibits PCa cell growth. MQ-mediated ROS simultaneously downregulated Akt phosphorylation and activated extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and adenosine monophosphate-activated protein kinase (AMPK) signaling in PC3 cells [2]. in vivo: Pregnant rats were treated orally with AS (15 and 40 mg/kg body weight (bwt)/day), MQ (30 and 80 mg/kg bwt/day) and AS/MQ (15/30 and 40/80 mg/kg bwt/day) on days 9-11 post coitum (pc). The dams were euthanized on day 12 pc and gestational and embryos histological parameters were evaluated [3]. Clinical trial: Activity of Mefloquine Against Urinary Schistosomiasis . Phase 2
- Valechlorine
Catalog No.:BCN2763
CAS No.:51771-49-4
- Estra-4,9-diene-3,17-dione
Catalog No.:BCC8959
CAS No.:5173-46-6
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Shikonine
Catalog No.:BCN3530
CAS No.:517-89-5
- Shikonin
Catalog No.:BCN1006
CAS No.:517-88-4
- Dicentrine
Catalog No.:BCN3296
CAS No.:517-66-8
- Stephanine
Catalog No.:BCN5643
CAS No.:517-63-5
- Corytuberine
Catalog No.:BCN2670
CAS No.:517-56-6
- Sennidin B
Catalog No.:BCN6355
CAS No.:517-44-2
- Hematoxylin
Catalog No.:BCC5322
CAS No.:517-28-2
- 2-Acetylbutyrolactone
Catalog No.:BCC8515
CAS No.:517-23-7
- Kadsurin
Catalog No.:BCN3634
CAS No.:51670-40-7
- Carteolol HCl
Catalog No.:BCC6466
CAS No.:51781-21-6
- Rengynic acid
Catalog No.:BCN5644
CAS No.:517883-38-4
- Dehydroglyasperin D
Catalog No.:BCN6829
CAS No.:517885-72-2
- Evodiamine
Catalog No.:BCN1092
CAS No.:518-17-2
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- (-)-beta-Peltatin
Catalog No.:BCN3606
CAS No.:518-29-6
- Tetrandrine
Catalog No.:BCN5955
CAS No.:518-34-3
- Corydaline
Catalog No.:BCN2342
CAS No.:518-69-4
- Emodin
Catalog No.:BCN5649
CAS No.:518-82-1
- Xanthopurpurin
Catalog No.:BCN6723
CAS No.:518-83-2
- Cycleanine
Catalog No.:BCN8445
CAS No.:518-94-5
- Isomaculosidine
Catalog No.:BCN7069
CAS No.:518-96-7
Asymmetric Synthesis of (+)-anti- and (-)-syn-Mefloquine Hydrochloride.[Pubmed:27657347]
J Org Chem. 2016 Oct 21;81(20):9567-9575.
The asymmetric (er > 99:1) total synthesis of (+)-anti- and (-)-syn-Mefloquine hydrochloride from a common intermediate is described. The Sharpless asymmetric dihydroxylation is the key asymmetric transformation used in the synthesis of this intermediate. It is carried out on an olefin that is accessed in three steps from commercially available materials, making the overall synthetic sequence very concise. The common diol intermediate derived from the Sharpless asymmetric dihydroxylation is converted into either a trans- or cis-epoxide, and these are subsequently converted to (+)-anti- and (-)-syn-mefloquine, respectively. X-ray crystallographic analysis of derivatives of (+)-anti- and (-)-syn-mefloquine is used to lay to rest a 40 year argument regarding the absolute stereochemistry of the mefloquines. A formal asymmetric (er > 99:1) synthesis of (+)-anti-Mefloquine hydrochloride is also presented that uses a Sharpless asymmetric epoxidation as a key step.
The interaction of mefloquine hydrochloride with cell membrane models at the air-water interface is modulated by the monolayer lipid composition.[Pubmed:24980622]
J Colloid Interface Sci. 2014 Oct 1;431:24-30.
The antiparasitic properties of antiparasitic drugs are believed to be associated with their interactions with the protozoan membrane, encouraging research on the identification of membrane sites capable of drug binding. In this study, we investigated the interaction of Mefloquine hydrochloride, known to be effective against malaria, with cell membrane models represented by Langmuir monolayers of selected lipids. It is shown that even small amounts of the drug affect the surface pressure-area isotherms as well as surface vibrational spectra of some lipid monolayers, which points to a significant interaction. The effects on the latter depend on the electrical charge of the monolayer-forming molecules, with the drug activity being particularly distinctive for negatively charged lipids. Therefore, the lipid composition of the monolayer modulates the interaction with the lipophilic drug, which may have important implications in understanding how the drug acts on specific sites of the protozoan membrane.
A Concise and Highly Enantioselective Total Synthesis of (+)-anti- and (-)-syn-Mefloquine Hydrochloride: Definitive Absolute Stereochemical Assignment of the Mefloquines.[Pubmed:26422780]
Angew Chem Int Ed Engl. 2015 Nov 16;54(47):14070-4.
A concise asymmetric (>99:1 e.r.) total synthesis of (+)-anti- and (-)-syn-Mefloquine hydrochloride from a common intermediate is described. The key asymmetric transformation is a Sharpless dihydroxylation of an olefin that is accessed in three steps from commercially available materials. The Sharpless-derived diol is converted into either a trans or cis epoxide, and these are subsequently converted into (+)-anti- and (-)-syn-mefloquine, respectively. The synthetic (+)-anti- and (-)-syn-mefloquine samples were derivatized with (S)-(+)-mandelic acid tert-butyldimethylsilyl ether, and a crystal structure of each derivative was obtained. These are the first X-ray structures for mefloquine derivatives that were obtained by coupling to a known chiral, nonracemic compound, and provide definitive confirmation of the absolute stereochemistry of (+)-anti- as well as (-)-syn-mefloquine.
Evaluation of performance of co crystals of mefloquine hydrochloride in tablet dosage form.[Pubmed:22639963]
Drug Dev Ind Pharm. 2013 May;39(5):716-23.
OBJECTIVE: The objective of present investigation was to evaluate performance of cocrystals of Mefloquine hydrochloride (MFL) in tablet dosage form. Our previous investigation showed significant effect of cocrystal formers on improving the solubility and dissolution rate of Mefloquine hydrochloride by cocrystallization method when prepared by solution cocrystallization method. MATERIALS AND METHODS: Prepared cocrystals of MFL with different ratio of cocrystal formers were incorporated in tablet dosage form and evaluated for micrometric properties, drug content, hardness, disintegration test, vitro dissolution studies and stability studies. Performance was compared with laboratory prepared tablet of MFL 250 mg. RESULTS: The considerable improvement in the dissolution rate was observed in case of cocrystals based tablets than pure MFL tablets. DISCUSSION AND CONCLUSION: So we can incorporate cocrystals in tablet dosage form to enhance in vitro and in vivo performance. To the best of our knowledge, this is the first report, cocrystals has been evaluated in tablet dosage form.


