TetrandrineCAS# 518-34-3 |
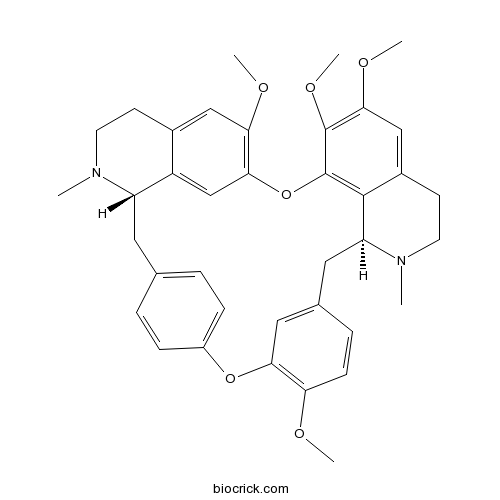
- Isotetrandrine
Catalog No.:BCN5538
CAS No.:477-57-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 518-34-3 | SDF | Download SDF |
| PubChem ID | 73078 | Appearance | White-pale yellow powder |
| Formula | C38H42N2O6 | M.Wt | 622.76 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Fanchinine; Hanfangchin A; Sinomenine A | ||
| Solubility | DMSO : 5 mg/mL (8.03 mM; Need ultrasonic and warming) H2O : < 0.1 mg/mL (insoluble) | ||
| SMILES | CN1CCC2=CC(=C3C=C2C1CC4=CC=C(C=C4)OC5=C(C=CC(=C5)CC6C7=C(O3)C(=C(C=C7CCN6C)OC)OC)OC)OC | ||
| Standard InChIKey | WVTKBKWTSCPRNU-KYJUHHDHSA-N | ||
| Standard InChI | InChI=1S/C38H42N2O6/c1-39-15-13-25-20-32(42-4)34-22-28(25)29(39)17-23-7-10-27(11-8-23)45-33-19-24(9-12-31(33)41-3)18-30-36-26(14-16-40(30)2)21-35(43-5)37(44-6)38(36)46-34/h7-12,19-22,29-30H,13-18H2,1-6H3/t29-,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tetrandrine is a calcium channel blocker, which shows antitumor, antifibrotic, anti-oxidant, anti-inflammatory and immunosuppressive activity. It suppressed Wnt/β-catenin signaling transduction, the migration of DU145 and PC-3 cells, EOMA cell growth through the ROS/Akt pathway and inhibited inward rectifying potassium current in cultured bovine aortic endothelial cells. |
| Targets | Caspase | Akt | Bcl-2/Bax | PI3K | TNF-α | TGF-β/Smad | ROS | CDK | p53 | p21 |
| In vitro | Tetrandrine suppresses proliferation, induces apoptosis, and inhibits migration and invasion in human prostate cancer cells.[Pubmed: 25677131]Asian J Androl. 2015 Feb 6.Tetrandrine (TET), a traditional Chinese medicine, exerts remarkable anticancer activity on various cancer cells. However, little is known about the effect of TET on human prostate cancer cells, and the mechanism of function of TET on prostate cancer has not yet been elucidated.
Tetrandrine inhibits inward rectifying potassium current in cultured bovine aortic endothelial cells.[Pubmed: 11603285]Acta Pharmacol Sin. 2000 Dec;21(12):1115-8.To study the effect of Tetrandrine (Tet) on inward rectifying potassium current in cultured bovine aortic endothelial cells.
|
| In vivo | Antifibrotic effects of tetrandrine on hepatic stellate cells and rats with liver fibrosis.[Pubmed: 17201889 ]J Gastroenterol Hepatol. 2007 Jan;22(1):99-111.Tetrandrine (TET), a traditional Chinese medicine, exerts remarkable anticancer activity on various cancer cells. However, little is known about the effect of TET on human prostate cancer cells, and the mechanism of function of TET on prostate cancer has not yet been elucidated.
|
| Cell Research | Tetrandrine induces early G1 arrest in human colon carcinoma cells by down-regulating the activity and inducing the degradation of G1-S-specific cyclin-dependent kinases and by inducing p53 and p21Cip1.[Pubmed: 15604277]Cancer Res. 2004 Dec 15;64(24):9086-92.Tetrandrine is an antitumor alkaloid isolated from the root of Stephania tetrandra.
|
| Animal Research | Tetrandrine induces G1/S cell cycle arrest through the ROS/Akt pathway in EOMA cells and inhibits angiogenesis in vivo.[Pubmed: 25355542]Int J Oncol. 2015 Jan;46(1):360-8.Tetrandrine, a bisbenzylisoquinoline alkaloid, is known to inhibit tumor cell proliferation and induce apoptosis in cancer models in vitro and in vivo.
|
| Structure Identification | Int J Immunopharmacol. 1989;11(4):395-401.Anti-inflammatory and immunosuppressive properties of the bis-benzylisoquinolines: in vitro comparisons of tetrandrine and berbamine.[Pubmed: 2777433]Tetrandrine and berbamine are two naturally occurring analogues with a bis-benzylisoquinoline structure. Comparative in vitro studies show that Tetrandrine has significantly greater suppressive effects on adherence, locomotion and 3H-deoxyglucose uptake of neutrophils, as well as the mitogen-induced lymphocyte responses and mixed lymphocyte reactions. Also, Tetrandrine displayed anti-oxidant activity while berbamine did not. By contrast, berbamine demonstrated a significantly greater capacity for inhibition of NK cell cytotoxicity. These results show that Tetrandrine is superior to berbamine in most aspects of anti-inflammatory and immunosuppressive activity. Since these two alkaloids differ by only one substitution in the side chain of one of the benzene rings, these findings may provide further insight into structure-activity relationships and clues to the synthesis and development of active analogues of this promising class of drugs for the treatment of chronic inflammatory diseases. |

Tetrandrine Dilution Calculator

Tetrandrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6058 mL | 8.0288 mL | 16.0576 mL | 32.1151 mL | 40.1439 mL |
| 5 mM | 0.3212 mL | 1.6058 mL | 3.2115 mL | 6.423 mL | 8.0288 mL |
| 10 mM | 0.1606 mL | 0.8029 mL | 1.6058 mL | 3.2115 mL | 4.0144 mL |
| 50 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6423 mL | 0.8029 mL |
| 100 mM | 0.0161 mL | 0.0803 mL | 0.1606 mL | 0.3212 mL | 0.4014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-beta-Peltatin
Catalog No.:BCN3606
CAS No.:518-29-6
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- Evodiamine
Catalog No.:BCN1092
CAS No.:518-17-2
- Dehydroglyasperin D
Catalog No.:BCN6829
CAS No.:517885-72-2
- Rengynic acid
Catalog No.:BCN5644
CAS No.:517883-38-4
- Carteolol HCl
Catalog No.:BCC6466
CAS No.:51781-21-6
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
- Valechlorine
Catalog No.:BCN2763
CAS No.:51771-49-4
- Estra-4,9-diene-3,17-dione
Catalog No.:BCC8959
CAS No.:5173-46-6
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Shikonine
Catalog No.:BCN3530
CAS No.:517-89-5
- Shikonin
Catalog No.:BCN1006
CAS No.:517-88-4
- Corydaline
Catalog No.:BCN2342
CAS No.:518-69-4
- Emodin
Catalog No.:BCN5649
CAS No.:518-82-1
- Xanthopurpurin
Catalog No.:BCN6723
CAS No.:518-83-2
- Cycleanine
Catalog No.:BCN8445
CAS No.:518-94-5
- Isomaculosidine
Catalog No.:BCN7069
CAS No.:518-96-7
- 3,3'-Di-O-methylellagic acid 4'-glucoside
Catalog No.:BCN1431
CAS No.:51803-68-0
- Nimesulide
Catalog No.:BCC4435
CAS No.:51803-78-2
- Oxoepistephamiersine
Catalog No.:BCN5645
CAS No.:51804-68-3
- Dihydrooxoepistephamiersine
Catalog No.:BCN5646
CAS No.:51804-69-4
- Raltegravir (MK-0518)
Catalog No.:BCC2137
CAS No.:518048-05-0
- KX1-004
Catalog No.:BCC5440
CAS No.:518058-84-9
- 2',4'-Dihydroxychalcone
Catalog No.:BCN5647
CAS No.:1776-30-3
Tetrandrine inhibits inward rectifying potassium current in cultured bovine aortic endothelial cells.[Pubmed:11603285]
Acta Pharmacol Sin. 2000 Dec;21(12):1115-8.
AIM: To study the effect of Tetrandrine (Tet) on inward rectifying potassium current in cultured bovine aortic endothelial cells. METHODS: Inward rectifying potassium current (IRK) was observed by the whole cell patch-clamp technique. RESULTS: IRK was inhibited by Tet in a concentration-dependent manner and recovered to normal after wash with drug-free external solution. IRK was reduced from (582 +/- 48) pA to (221 +/- 40) pA at a holding potential of -70 mV by Tet 30 mumol/L. IC50 was 2.8 mumol/L. CONCLUSION: Tet inhibited inward rectifying potassium current in cultured bovine aortic endothelial cells.
Antifibrotic effects of tetrandrine on hepatic stellate cells and rats with liver fibrosis.[Pubmed:17201889]
J Gastroenterol Hepatol. 2007 Jan;22(1):99-111.
BACKGROUND: Anti-inflammation strategies are one of the proposed therapeutic approaches to hepatic fibrosis. Tetrandrine (C(38)H(42)O(8)N(2), molecular weight: 622; Tet), an alkaloid isolated from the Chinese medicinal herb Stephania tetrandra, has been shown to exert anti-inflammatory activity in pulmonary diseases. The purpose of the present study was to investigate the in vitro and in vivo effects of Tet on hepatic fibrosis. METHODS: A cell line of rat hepatic stellate cells (HSC-T6) was stimulated with transforming growth factor-beta1 (TGF-beta1) or tumor necrosis factor-alpha (TNF-alpha). The inhibitory effects of Tet on the nuclear factor kappaB (NFkappaB) signaling cascade and molecular markers including intercellular adhesion molecule-1 (ICAM-1) and alpha-smooth muscle actin (alpha-SMA) secretion were assessed. Fibrosis was induced by dimethylnitrosamine (DMN) administration in rats for 4 weeks. Fibrotic rats were randomly assigned to one of the four groups: vehicle (0.7% carboxyl methyl cellulose, CMC), Tet (1 mg/kg), Tet (5 mg/kg), or silymarin (50 mg/kg), each given by gavage twice daily for 3 weeks starting after 1 week of DMN administration. At the end of the study, liver tissues were scored for fibrosis and analyzed for molecular markers of fibrosis. RESULTS: Tetrandrine (0.5-5.0 micromol/L) concentration-dependently inhibited NFkappaB transcriptional activity induced by TNF-alpha, including IkappaBalpha phosphorylation and mRNA expressions of ICAM-1 in HSC-T6 cells. In addition, Tet also inhibited TGF-beta1-induced alpha-SMA secretion and collagen deposition in HSC-T6 cells. Fibrosis scores of livers from DMN-treated rats with high-dose Tet (1.3 +/- 0.3) were significantly reduced in comparison with DMN-treated rats receiving saline (2.0 +/- 0.2). Hepatic collagen content of DMN rats was significantly reduced by either Tet or silymarin treatment. Double-staining results showed that alpha-SMA- and NFkappaB-positive cells were decreased in the fibrotic livers by Tet and silymarin treatment. In addition, mRNA expression of ICAM-1, alpha-SMA, and TGF-beta1 was attenuated by Tet treatment. Moreover, levels of plasma aspartate aminotransferase and alanine aminotransferase activities were reduced by Tet and silymarin treatment. CONCLUSION: Tetrandrine exerts antifibrotic effects in both HSC-T6 cells and in rats with DMN-induced fibrosis.
Tetrandrine induces early G1 arrest in human colon carcinoma cells by down-regulating the activity and inducing the degradation of G1-S-specific cyclin-dependent kinases and by inducing p53 and p21Cip1.[Pubmed:15604277]
Cancer Res. 2004 Dec 15;64(24):9086-92.
Tetrandrine is an antitumor alkaloid isolated from the root of Stephania tetrandra. We find that micromolar concentrations of Tetrandrine irreversibly inhibit the proliferation of human colon carcinoma cells in MTT and clonogenic assays by arresting cells in G(1). Tetrandrine induces G(1) arrest before the restriction point in nocodazole- and serum-starved synchronized HT29 cells, without affecting the G(1)-S transition in aphidicolin-synchronized cells. Tetrandrine-induced G(1) arrest is followed by apoptosis as shown by fluorescence-activated cell sorting, terminal deoxynucleotidyl transferase-mediated nick end labeling, and annexin V staining assays. Tetrandrine-induced early G(1) arrest is mediated by at least three different mechanisms. First, Tetrandrine inhibits purified cyclin-dependent kinase 2 (CDK2)/cyclin E and CDK4 without affecting significantly CDK2/cyclin A, CDK1/cyclin B, and CDK6. Second, Tetrandrine induces the proteasome-dependent degradation of CDK4, CDK6, cyclin D1, and E2F1. Third, Tetrandrine increases the expression of p53 and p21(Cip1) in wild-type p53 HCT116 cells. Collectively, these results show that Tetrandrine arrests cells in G(1) by convergent mechanisms, including down-regulation of E2F1 and up-regulation of p53/p21(Cip1).
Tetrandrine suppresses proliferation, induces apoptosis, and inhibits migration and invasion in human prostate cancer cells.[Pubmed:25677131]
Asian J Androl. 2015 Sep-Oct;17(5):850-3.
Tetrandrine (TET), a traditional Chinese medicine, exerts remarkable anticancer activity on various cancer cells. However, little is known about the effect of TET on human prostate cancer cells, and the mechanism of function of TET on prostate cancer has not yet been elucidated. To investigate the effects of TET on the suppression of proliferation, induction of apoptosis, and inhibition of migration and invasion in human prostate cancer cell lines, DU145 and PC-3. Inhibition of growth was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and clone formation assay, and flow cytometry analysis was performed to detect the induction of apoptosis. Activation of poly (ADP-ribose) polymerase, caspase-3, Akt, phospho-Akt, Bcl-2, and Bax was analyzed by Western blotting. Wound healing assay and transwell migration assay were used to evaluate the effect of TET on migration and invasion of cancer cells. TET inhibited the growth of DU145 and PC-3 cells in a dose- and time-dependent manner. Cell cloning was inhibited in the presence of TET in DU145 and PC-3 cells. TET suppressed the migration of DU145 and PC-3 cells. Transwell invasion assay showed that TET significantly weakened invasion capacity of DU145 and PC-3 cells. TET exhibited strong inhibitory effect on proliferation, migration, and invasion of prostate cancer cells. In addition, TET induced apoptosis in a dose-dependent manner by activating the caspase cascade and inhibiting phosphoinositide 3-kinase-Akt signal pathway. The accumulating evidence suggests that TET could be a potential therapeutic candidate against prostate cancer in a clinical setting.
Tetrandrine induces G1/S cell cycle arrest through the ROS/Akt pathway in EOMA cells and inhibits angiogenesis in vivo.[Pubmed:25355542]
Int J Oncol. 2015 Jan;46(1):360-8.
Tetrandrine, a bisbenzylisoquinoline alkaloid, is known to inhibit tumor cell proliferation and induce apoptosis in cancer models in vitro and in vivo. In the present study, Tetrandrine significantly inhibited the proliferation of mouse endothelial cells (EOMA cell) and induced G1/S arrest in EOMA cells, in which the expressions of cyclin D and cyclin E and CDKs were downregulated. Tetrandrine treatment also caused intracellular accumulation of reactive oxygen species (ROS). Pretreatment with NAC, which is a ROS inhibitor, blocked G1/S cell arrest and cyclin regulation induced by Tetrandrine, implying that ROS generation plays an important role in Tetrandrine-induced cell cycle arrest. Furthermore, a decreased phospho-Akt protein level after Tetrandrine treatment was reversible with the removal of the intracellular ROS by NAC. Notably, overexpression of Akt decreased Tetrandrine-induced G1/S arrest. Finally, we verified the antiangiogenic effects of Tetrandrine in vivo in a liver cancer xenograft model in nude mice. In conclusion, Tetrandrine inhibits EOMA cell growth through the ROS/Akt pathway, and it could be a promising compound for cancer therapy as an inhibitor of tumor vascular growth.
Anti-inflammatory and immunosuppressive properties of the bis-benzylisoquinolines: in vitro comparisons of tetrandrine and berbamine.[Pubmed:2777433]
Int J Immunopharmacol. 1989;11(4):395-401.
Tetrandrine and berbamine are two naturally occurring analogues with a bis-benzylisoquinoline structure. Comparative in vitro studies show that Tetrandrine has significantly greater suppressive effects on adherence, locomotion and 3H-deoxyglucose uptake of neutrophils, as well as the mitogen-induced lymphocyte responses and mixed lymphocyte reactions. Also, Tetrandrine displayed anti-oxidant activity while berbamine did not. By contrast, berbamine demonstrated a significantly greater capacity for inhibition of NK cell cytotoxicity. These results show that Tetrandrine is superior to berbamine in most aspects of anti-inflammatory and immunosuppressive activity. Since these two alkaloids differ by only one substitution in the side chain of one of the benzene rings, these findings may provide further insight into structure-activity relationships and clues to the synthesis and development of active analogues of this promising class of drugs for the treatment of chronic inflammatory diseases.


