ShikonineCAS# 517-89-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 517-89-5 | SDF | Download SDF |
| PubChem ID | 479503 | Appearance | Brown powder |
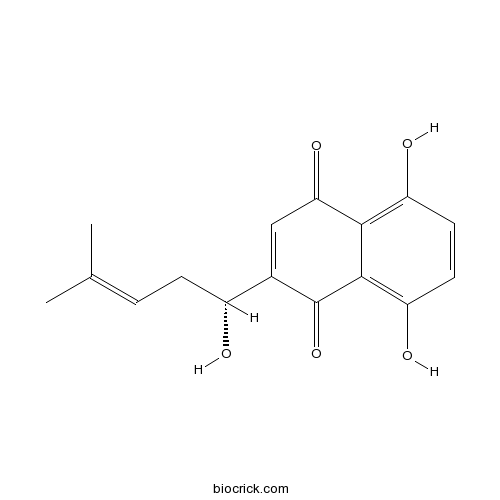
| Formula | C16H16O5 | M.Wt | 288.3 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Synonyms | C.I. 75535; Isoarnebin 4;shikonin;(-)-Shikonin | ||
| Solubility | DMSO : ≥ 31 mg/mL (107.53 mM); | ||
| Chemical Name | 5,8-dihydroxy-2-[(1R)-1-hydroxy-4-methylpent-3-enyl]naphthalene-1,4-dione | ||
| SMILES | CC(=CCC(C1=CC(=O)C2=C(C=CC(=C2C1=O)O)O)O)C | ||
| Standard InChIKey | NEZONWMXZKDMKF-SNVBAGLBSA-N | ||
| Standard InChI | InChI=1S/C16H16O5/c1-8(2)3-4-10(17)9-7-13(20)14-11(18)5-6-12(19)15(14)16(9)21/h3,5-7,10,17-19H,4H2,1-2H3/t10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Shikonin(Shikonine), a potent and specific Pyruvate kinase M2 (PKM2) inhibitor, has antibacterial, antitumor, and anti-inflammatory effects, it provides neuroprotection by reducing the release of various proinflammatory molecules from activated microglia. Shikonin can inhibit VEGF-induced angiogenesis and suppress tumor growth in lewis lung carcinoma-bearing mice. |
| Targets | Antifection | VEGFR | PKM2 | Cdk4 | HIV | CXCR4 |
| In vitro | Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2.[Pubmed: 21516121]Oncogene, 2011, 30(42):4297-306.We recently reported that shikonin(Shikonine) and its analogs were a class of necroptotic inducers that could bypass cancer drug resistance. However, the molecular targets of shikonin are not known. Shikonin Inhibits Intestinal Calcium-Activated Chloride Channels and Prevents Rotaviral Diarrhea.[Pubmed: 27601995 ]Front Pharmacol. 2016 Aug 23;7:270.Secretory diarrhea remains a global health burden and causes major mortality in children. There have been some focuses on antidiarrheal therapies that may reduce fluid losses and intestinal motility in diarrheal diseases. |
| In vivo | Shikonin Suppresses Skin Carcinogenesis via Inhibiting Cell Proliferation.[Pubmed: 25961580 ]PLoS One. 2015 May 11;10(5):e0126459.The M2 isoform of pyruvate kinase M2 (PKM2) has been shown to be up-regulated in human skin cancers. |
| Kinase Assay | Shikonin, a component of chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1.[Pubmed: 12936978 ]Antimicrob Agents Chemother. 2003 Sep;47(9):2810-6.Shikonin(Shikonine) is a major component of zicao (purple gromwell, the dried root of Lithospermum erythrorhizon), a Chinese herbal medicine with various biological activities, including inhibition of human immunodeficiency virus (HIV) type 1 (HIV-1). G protein-coupled chemokine receptors are used by HIV-1 as coreceptors to enter the host cells. |

Shikonine Dilution Calculator

Shikonine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4686 mL | 17.343 mL | 34.6861 mL | 69.3722 mL | 86.7152 mL |
| 5 mM | 0.6937 mL | 3.4686 mL | 6.9372 mL | 13.8744 mL | 17.343 mL |
| 10 mM | 0.3469 mL | 1.7343 mL | 3.4686 mL | 6.9372 mL | 8.6715 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6937 mL | 1.3874 mL | 1.7343 mL |
| 100 mM | 0.0347 mL | 0.1734 mL | 0.3469 mL | 0.6937 mL | 0.8672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Shikonin is an inhibitor of TMEM16A chloride channel with an IC50 of 6.5 μM. Shikonin is also a specific inhibitor of PKM2 and can also inhibit tumor necrosis factor-α (TNF-α) and prevent activation of nuclear factor-κB (NF-κB) pathway.
In Vitro:Shikonin is an inhibitor of TMEM16A chloride channel with an IC50 of 6.5 μM[1]. Shikonin is also a specific inhibitor of PKM2[2] and can also inhibit tumor necrosis factor-α (TNF-α) and prevent activation of nuclear factor-κB (NF-κB) pathway. Shikonin at concentrations higher than 50 μM significantly inhibits ormal human keratinocytes (NHKs) viability, compare with that of control (P<0.05). Pretreatment with Shikonin for 2 h attenuates TNF-α-induced NF-κB p65 nuclear translocation[3]. Treatments of Shikonin at 5 and 7.5 μM significantly inhibit the cell viability starting from 12 h and the inhibitory effects are presented in time-dependent patterns compare with the 0 h group in both cell lines. It is found that 5 μM Shikonin displays greater inhibition compare to 2.5 μM at the time points from 24 to 48 h. The invasiveness of U87 and U251 cells is significantly attenuated when treated with Shikonin at 2.5, 5, and 7.5 μM compare with the control group at 24 and 48 h (p<0.01)[4].
In Vivo:Shikonin significantly inhibits the increase in IL-1β and TNF-α expression levels in the rat model of osteoarthritis, compare with those in the osteoarthritis group (P<0.01). The NF-κB protein expression level is significantly suppressed by Shikonin in the rat model of osteoarthritis, compare with that in the osteoarthritis group (P<0.01). The induction of the iNOS level is suppressed by treatment with Shikonin in the rat model of osteoarthritis, compare with that in the osteoarthritis group (P<0.01). The administration of Shikonin markedly weakens the up-regulation of COX-2 protein expression in the rat model of osteoarthritis, as compare with that in the osteoarthritis group (P<0.01). The elevation of caspase-3 activity is significantly reduced by Shikonin treatment in the rat model of osteoarthritis, compare with that in the osteoarthritis group (P<0.01). The downregulation of Akt phosphorylation is also significantly recovered by treatment with Shikonin in the rat model of osteoarthritis, compare with that in the osteoarthritis group (P<0.01)[5].
References:
[1]. Jiang Y et al. Shikonin Inhibits Intestinal Calcium-Activated Chloride Channels and Prevents Rotaviral Diarrhea. Front Pharmacol. 2016 Aug 23;7:270.
[2]. Li W, et al. Shikonin Suppresses Skin Carcinogenesis via Inhibiting Cell Proliferation. PLoS One. 2015 May 11;10(5):e0126459.
[3]. Yan Y, et al. Shikonin Promotes Skin Cell Proliferation and Inhibits Nuclear Factor-κB Translocation via Proteasome Inhibition In Vitro. Chin Med J (Engl). 2015 Aug 20;128(16):2228-33.
[4]. Zhang FY, et al. Shikonin Inhibits the Migration and Invasion of Human Glioblastoma Cells by Targeting Phosphorylated β-Catenin and Phosphorylated PI3K/Akt: A Potential Mechanism for the Anti-Glioma Efficacy of a Traditional Chinese Herbal Medicine. Int J Mol Sci. 2015 Oct 9;16(10):23823-48.
[5]. Fu D, et al. Shikonin inhibits inflammation and chondrocyte apoptosis by regulation of the PI3K/Akt signaling pathway in a rat model of osteoarthritis. Exp Ther Med. 2016 Oct;12(4):2735-2740.
- Shikonin
Catalog No.:BCN1006
CAS No.:517-88-4
- Dicentrine
Catalog No.:BCN3296
CAS No.:517-66-8
- Stephanine
Catalog No.:BCN5643
CAS No.:517-63-5
- Corytuberine
Catalog No.:BCN2670
CAS No.:517-56-6
- Sennidin B
Catalog No.:BCN6355
CAS No.:517-44-2
- Hematoxylin
Catalog No.:BCC5322
CAS No.:517-28-2
- 2-Acetylbutyrolactone
Catalog No.:BCC8515
CAS No.:517-23-7
- Kadsurin
Catalog No.:BCN3634
CAS No.:51670-40-7
- Erythrartine
Catalog No.:BCN5642
CAS No.:51666-26-3
- 2-Methoxyphenalen-1-one
Catalog No.:BCN7181
CAS No.:51652-39-2
- Murrangatin diacetate
Catalog No.:BCN5641
CAS No.:51650-59-0
- BML-277
Catalog No.:BCC4245
CAS No.:516480-79-8
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Estra-4,9-diene-3,17-dione
Catalog No.:BCC8959
CAS No.:5173-46-6
- Valechlorine
Catalog No.:BCN2763
CAS No.:51771-49-4
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
- Carteolol HCl
Catalog No.:BCC6466
CAS No.:51781-21-6
- Rengynic acid
Catalog No.:BCN5644
CAS No.:517883-38-4
- Dehydroglyasperin D
Catalog No.:BCN6829
CAS No.:517885-72-2
- Evodiamine
Catalog No.:BCN1092
CAS No.:518-17-2
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- (-)-beta-Peltatin
Catalog No.:BCN3606
CAS No.:518-29-6
- Tetrandrine
Catalog No.:BCN5955
CAS No.:518-34-3
- Corydaline
Catalog No.:BCN2342
CAS No.:518-69-4
Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2.[Pubmed:21516121]
Oncogene. 2011 Oct 20;30(42):4297-306.
We recently reported that shikonin and its analogs were a class of necroptotic inducers that could bypass cancer drug resistance. However, the molecular targets of shikonin are not known. Here, we showed that shikonin and its analogs are inhibitors of tumor-specific pyruvate kinase-M2 (PKM2), among which shikonin and its enantiomeric isomer alkannin were the most potent and showed promising selectivity, that is, shikonin and alkannin at concentrations that resulted in over 50% inhibition of PKM2 activity did not inhibit PKM1 and pyruvate kinase-L (PKL). Shikonin and alkannin significantly inhibited the glycolytic rate, as manifested by cellular lactate production and glucose consumption in drug-sensitive and resistant cancer cell lines (MCF-7, MCF-7/Adr, MCF-7/Bcl-2, MCF-7/Bcl-x(L) and A549) that primarily express PKM2. HeLa cells transfected with PKM1 showed reduced sensitivity to shikonin- or alkannin-induced cell death. To the best of our knowledge, shikonin and alkannin are the most potent and specific inhibitors to PKM2 reported so far. As PKM2 universally expresses in cancer cells and dictates the last rate-limiting step of glycolysis vital for cancer cell proliferation and survival, enantiomeric shikonin and alkannin may have potential in future clinical application.


