(-)-beta-PeltatinCAS# 518-29-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 518-29-6 | SDF | Download SDF |
| PubChem ID | 92122 | Appearance | Powder |
| Formula | C22H22O8 | M.Wt | 414.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
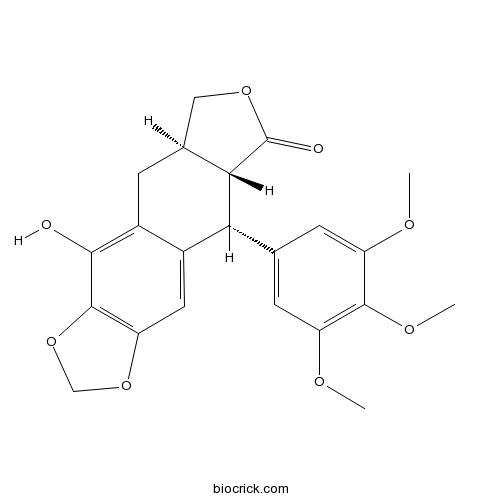
| Chemical Name | (5aR,8aR,9R)-4-hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one | ||
| SMILES | COC1=CC(=CC(=C1OC)OC)C2C3C(CC4=C(C5=C(C=C24)OCO5)O)COC3=O | ||
| Standard InChIKey | HLBPOYVRLSXWJJ-PDSMFRHLSA-N | ||
| Standard InChI | InChI=1S/C22H22O8/c1-25-14-5-10(6-15(26-2)20(14)27-3)17-12-7-16-21(30-9-29-16)19(23)13(12)4-11-8-28-22(24)18(11)17/h5-7,11,17-18,23H,4,8-9H2,1-3H3/t11-,17+,18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (-)-beta-Peltatin has antimitotic, and antineoplastic activities. |

(-)-beta-Peltatin Dilution Calculator

(-)-beta-Peltatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4131 mL | 12.0656 mL | 24.1313 mL | 48.2625 mL | 60.3282 mL |
| 5 mM | 0.4826 mL | 2.4131 mL | 4.8263 mL | 9.6525 mL | 12.0656 mL |
| 10 mM | 0.2413 mL | 1.2066 mL | 2.4131 mL | 4.8263 mL | 6.0328 mL |
| 50 mM | 0.0483 mL | 0.2413 mL | 0.4826 mL | 0.9653 mL | 1.2066 mL |
| 100 mM | 0.0241 mL | 0.1207 mL | 0.2413 mL | 0.4826 mL | 0.6033 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- Evodiamine
Catalog No.:BCN1092
CAS No.:518-17-2
- Dehydroglyasperin D
Catalog No.:BCN6829
CAS No.:517885-72-2
- Rengynic acid
Catalog No.:BCN5644
CAS No.:517883-38-4
- Carteolol HCl
Catalog No.:BCC6466
CAS No.:51781-21-6
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
- Valechlorine
Catalog No.:BCN2763
CAS No.:51771-49-4
- Estra-4,9-diene-3,17-dione
Catalog No.:BCC8959
CAS No.:5173-46-6
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Shikonine
Catalog No.:BCN3530
CAS No.:517-89-5
- Shikonin
Catalog No.:BCN1006
CAS No.:517-88-4
- Dicentrine
Catalog No.:BCN3296
CAS No.:517-66-8
- Tetrandrine
Catalog No.:BCN5955
CAS No.:518-34-3
- Corydaline
Catalog No.:BCN2342
CAS No.:518-69-4
- Emodin
Catalog No.:BCN5649
CAS No.:518-82-1
- Xanthopurpurin
Catalog No.:BCN6723
CAS No.:518-83-2
- Cycleanine
Catalog No.:BCN8445
CAS No.:518-94-5
- Isomaculosidine
Catalog No.:BCN7069
CAS No.:518-96-7
- 3,3'-Di-O-methylellagic acid 4'-glucoside
Catalog No.:BCN1431
CAS No.:51803-68-0
- Nimesulide
Catalog No.:BCC4435
CAS No.:51803-78-2
- Oxoepistephamiersine
Catalog No.:BCN5645
CAS No.:51804-68-3
- Dihydrooxoepistephamiersine
Catalog No.:BCN5646
CAS No.:51804-69-4
- Raltegravir (MK-0518)
Catalog No.:BCC2137
CAS No.:518048-05-0
- KX1-004
Catalog No.:BCC5440
CAS No.:518058-84-9
Formation of beta-peltatin-A methyl ether and coniferin by root cultures of Linum flavum.[Pubmed:3760883]
J Nat Prod. 1986 May-Jun;49(3):435-9.
Extracts of root cultures of Linum flavum contained high cytotoxic activity due to the presence of 1% beta-peltatin-A methyl ether of the dry mass. During chromatographic analysis of the cell extracts, coniferin was identified as the major uv-absorbing but noncytotoxic constituent with levels of up to 3% of the dry mass. Growth, culture appearance, and product accumulation varied greatly with the 2,4-D concentration in the medium.
First synthesis and pharmacological evaluation of benzoindolizidine and benzoquinolizidine analogues of alpha- and beta-peltatin.[Pubmed:11003157]
Bioorg Med Chem. 2000 Aug;8(8):2113-25.
The benzoindolizidine and quinolizidine analogues of alpha- and beta-peltatin were designed and synthesized by two different synthetic routes involving as the key step the Bischler-Napieralski cyclization of suitably substituted N-acyl-2-arylmethylpyrrolidine and -piperidine derivatives. The in vitro biological activity of these analogues as well as some of their derivatives was subsequently evaluated.
Beta-peltatin 6-O-methyltransferase from suspension cultures of Linum nodiflorum.[Pubmed:12943762]
Phytochemistry. 2003 Sep;64(2):453-8.
S-Adenosyl-L-methionine:beta-peltatin 6-O-methyltransferase was isolated and characterized from cell suspension cultures of Linum nodiflorum L. (Linaceae), a Linum species accumulating aryltetralin lignans such as 6-methoxypodophyllotoxin. The enzyme transfers a methyl group from S-adenosyl-L-methionine to the only free OH-group of beta-peltatin in position 6 thus forming beta-peltatin-A methylether. This reaction is a putative biosynthetic step in the biosynthesis of 6-methoxypodophyllotoxin from deoxypodophyllotoxin. The enzyme has a pH-optimum at pH 7.7 and a temperature optimum at 40 degrees C. The enzyme activity is strongly inhibited by MnSO(4), FeCl(3), FeSO(4) and ZnSO(4) as well as S-adenosyl-homocysteine. Mg(2+) and EDTA did not influence the methylation of beta-peltatin. Substrate saturation curves were obtained for S-adenosyl-methionine and beta-peltatin and apparent K(m)-values of 15 microM and 40 microM, respectively, were determined for these substrates. Substrate inhibition was observed for beta-peltatin. No other lignan substrate tested nor caffeic acid were accepted. The suspension cell line of Linum nodiflorum was characterized with respect to growth, medium alterations and lignan production as well as activity of SAM:beta-peltatin 6-O-methyltransferase. Highest specific activities of beta-peltatin 6-O-methyltransferase were determined on day 7 of the culture period corresponding to the highest levels of 6-methoxypodophyllotoxin on days 7 to 12.


