3'-DemethylnobiletinCAS# 112448-39-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112448-39-2 | SDF | Download SDF |
| PubChem ID | 183466 | Appearance | Powder |
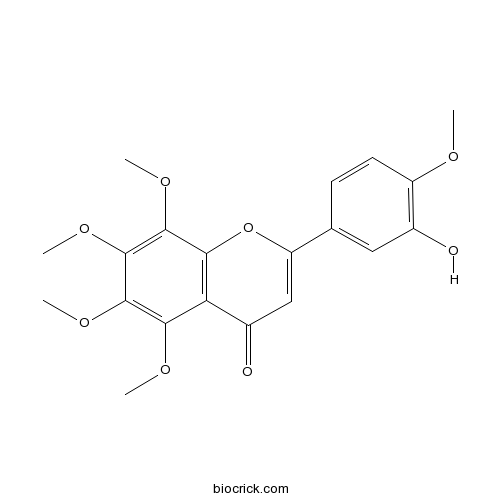
| Formula | C20H20O8 | M.Wt | 388.37 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3-hydroxy-4-methoxyphenyl)-5,6,7,8-tetramethoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=CC(=O)C3=C(O2)C(=C(C(=C3OC)OC)OC)OC)O | ||
| Standard InChIKey | XFYYZBJXMSDKCV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H20O8/c1-23-13-7-6-10(8-11(13)21)14-9-12(22)15-16(24-2)18(25-3)20(27-5)19(26-4)17(15)28-14/h6-9,21H,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 3'-Demethylnobiletin has chemopreventive effects on colon carcinogenesis, it can significantly inhibit the growth of human colon cancer cells, cause cell-cycle arrest, induce apoptosis, and profoundly modulate signaling proteins related with cell proliferation and cell death. 2. 3'-Demethylnobiletin significantly suppresses CD36 expression. |
| Targets | LDL | LOX | AP-1 | NF-kB |

3'-Demethylnobiletin Dilution Calculator

3'-Demethylnobiletin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5749 mL | 12.8743 mL | 25.7486 mL | 51.4973 mL | 64.3716 mL |
| 5 mM | 0.515 mL | 2.5749 mL | 5.1497 mL | 10.2995 mL | 12.8743 mL |
| 10 mM | 0.2575 mL | 1.2874 mL | 2.5749 mL | 5.1497 mL | 6.4372 mL |
| 50 mM | 0.0515 mL | 0.2575 mL | 0.515 mL | 1.0299 mL | 1.2874 mL |
| 100 mM | 0.0257 mL | 0.1287 mL | 0.2575 mL | 0.515 mL | 0.6437 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Erigoster B
Catalog No.:BCN8562
CAS No.:849777-61-3
- Acanthopanaxoside B
Catalog No.:BCN8561
CAS No.:915792-03-9
- Glabrolide
Catalog No.:BCN8560
CAS No.:10401-33-9
- Anemarrhenasaponin I
Catalog No.:BCN8559
CAS No.:163047-21-0
- Dihydropalmatine
Catalog No.:BCN8558
CAS No.:26067-60-7
- Rupestonic acid
Catalog No.:BCN8557
CAS No.:115473-63-7
- Uvarigranol B
Catalog No.:BCN8556
CAS No.:164204-79-9
- Ginsenoside Ra2
Catalog No.:BCN8555
CAS No.:83459-42-1
- Panasenoside
Catalog No.:BCN8554
CAS No.:31512-06-8
- Trans sodium crocetinate
Catalog No.:BCN8553
CAS No.:591230-99-8
- Ilexsaponin B2
Catalog No.:BCN8552
CAS No.:108906-69-0
- 28-Demethyl-beta-amyrone
Catalog No.:BCN8551
CAS No.:73493-60-4
- Lancifodilactone F
Catalog No.:BCN8564
CAS No.:850878-47-6
- Periplocoside N
Catalog No.:BCN8565
CAS No.:39946-41-3
- 3-Feruloyl-1-Sinapoyl sucrose
Catalog No.:BCN8566
CAS No.:98942-06-4
- Vinaginsenoside R8
Catalog No.:BCN8567
CAS No.:156042-22-7
- Saikogenin D
Catalog No.:BCN8568
CAS No.:5573-16-0
- Gancaonin N
Catalog No.:BCN8569
CAS No.:129145-52-4
- Graveobioside A
Catalog No.:BCN8570
CAS No.:506410-53-3
- 2-Amino-3-carboxy-1,4-naphthoquinone
Catalog No.:BCN8571
CAS No.:173043-38-4
- 4'-O-Methylochnaflavone
Catalog No.:BCN8572
CAS No.:49619-87-6
- Perisesaccharide B
Catalog No.:BCN8573
CAS No.:1095261-93-0
- Tectol
Catalog No.:BCN8574
CAS No.:24449-39-6
- Jionoside D
Catalog No.:BCN8575
CAS No.:120406-34-0
Suppressive effects of demethylated metabolites of nobiletin on phorbol ester-induced expression of scavenger receptor genes in THP-1 human monocytic cells.[Pubmed:18806314]
Biofactors. 2007;31(2):107-16.
Unregulated uptake of oxidized low-density lipoproteins (ox-LDL) via macrophage scavenger receptors (SRs) is a key event in atherosclerosis. We previously reported that nobiletin (NOB), a citrus polymethoxylated flavone, markedly reduced 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced SRs and adhesion molecules mRNA expression and blockade of DiI-acLDL uptake in THP-1 human monocyte-like cells. In this study, we examined the effects of NOB metabolites, 3'-hydroxy-5,6,7,8,4'-pentamethoxyflavone (3'-demethyl-NOB), 4'-hydroxy-5,6,7,8,3'-pentamethoxyflavone (4'-demethyl-NOB) and 3', 4'-dihydroxy-5,6,7,8,-tetramethoxyflavone (3', 4'-didemethyl-NOB) and NOB analog, tangeretin, on SRs and adhesion molecules mRNA expression. 3'-Demethyl-NOB significantly suppressed CD36 expression, moreover, 4'-demethyl- and 3', 4'-didemethyl-NOB significantly suppressed TPA-induced expression of SR-A and LOX-1. Further, the suppressive effects of 4'-demethyl- and 3', 4'-didemethyl-NOB on the expression of CD36 mRNA were greater extent than parent NOB. The inhibitory effects of the metabolites toward TPA-induced SR mRNA expression are partly associated with the suppression of AP-1 and NF-kappaB transcriptional activities. Together, our results suggest that metabolites of NOB, such as 4'-demethyl- and 3', 4'-didemethyl-NOB, have comparable or higher potentials to attenuate SR expression than NOB.
Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis.[Pubmed:26445322]
Mol Nutr Food Res. 2015 Dec;59(12):2383-94.
SCOPE: Nobiletin (NBT) is a major citrus flavonoid with various health benefits. Herein, we investigated the colon cancer chemopreventive effects of NBT and its colonic metabolites in a colitis-associated colon carcinogenesis mouse model as well as in human colon cancer cell models. METHODS AND RESULTS: In azoxymethane/dextran sulfate sodium treated mice, oral administration of NBT effectively decreased both incidence and multiplicity of colonic tumors. NBT showed significant antiproliferative, proapoptotic, and anti-inflammatory effects in the mouse colon. HPLC analysis revealed that oral administration of NBT resulted in high levels of metabolites, i.e. 3'-demethylnobiletin (M1), 4'-demethylnobiletin (M2), and 3',4'-didemethylnobiletin (M3) in the colonic mucosa. In contrast, the colonic level of NBT was about 20-fold lower than the total colonic level of three metabolites. Cell culture studies demonstrated that the colonic metabolites of NBT significantly inhibited the growth of human colon cancer cells, caused cell-cycle arrest, induced apoptosis, and profoundly modulated signaling proteins related with cell proliferation and cell death. All of these effects were much stronger than those produced by NBT alone. CONCLUSION: Our results demonstrated that oral administration of NBT significantly inhibited colitis-associated colon carcinogenesis in mice, and this chemopreventive effect was strongly associated with its colonic metabolites.


