Trans sodium crocetinateCAS# 591230-99-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 591230-99-8 | SDF | Download SDF |
| PubChem ID | 10287099 | Appearance | Powder |
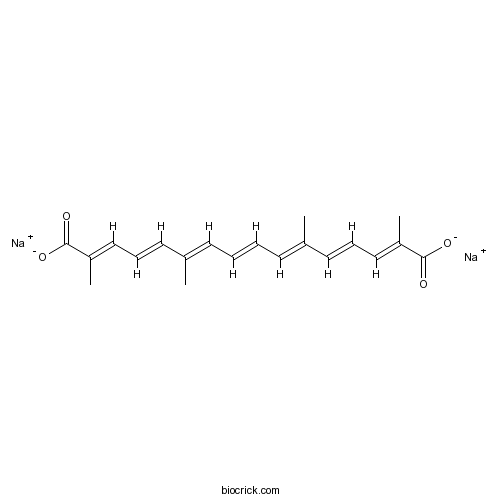
| Formula | C20H22Na2O4 | M.Wt | 372.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | disodium;(2E,4E,6E,8E,10E,12E,14E)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate | ||
| SMILES | CC(=CC=CC=C(C)C=CC=C(C)C(=O)[O-])C=CC=C(C)C(=O)[O-].[Na+].[Na+] | ||
| Standard InChIKey | RMDMBHQVNHQDDD-VFWKRBOSSA-L | ||
| Standard InChI | InChI=1S/C20H24O4.2Na/c1-15(11-7-13-17(3)19(21)22)9-5-6-10-16(2)12-8-14-18(4)20(23)24;;/h5-14H,1-4H3,(H,21,22)(H,23,24);;/q;2*+1/p-2/b6-5+,11-7+,12-8+,15-9+,16-10+,17-13+,18-14+;; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Trans sodium crocetinate (TSC) has been developed to enhance the delivery of oxygen to hypoxic tissues, TSC with temozolomide and radiation therapy for glioblastoma multiforme. 2. TSC provides neuroprotection against cerebral ischemia and reperfusion in obese mice. 3. TSC improves outcomes in rodent models of occlusive and hemorrhagic stroke. |
| Targets | MMP(e.g.TIMP) |

Trans sodium crocetinate Dilution Calculator

Trans sodium crocetinate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6853 mL | 13.4264 mL | 26.8528 mL | 53.7057 mL | 67.1321 mL |
| 5 mM | 0.5371 mL | 2.6853 mL | 5.3706 mL | 10.7411 mL | 13.4264 mL |
| 10 mM | 0.2685 mL | 1.3426 mL | 2.6853 mL | 5.3706 mL | 6.7132 mL |
| 50 mM | 0.0537 mL | 0.2685 mL | 0.5371 mL | 1.0741 mL | 1.3426 mL |
| 100 mM | 0.0269 mL | 0.1343 mL | 0.2685 mL | 0.5371 mL | 0.6713 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ilexsaponin B2
Catalog No.:BCN8552
CAS No.:108906-69-0
- 28-Demethyl-beta-amyrone
Catalog No.:BCN8551
CAS No.:73493-60-4
- Vinaginsenoside R3
Catalog No.:BCN8550
CAS No.:156012-92-9
- Cistanoside F
Catalog No.:BCN8549
CAS No.:97411-47-7
- Rhein-8-glucoside
Catalog No.:BCN8548
CAS No.:34298-86-7
- Dihydroartemisinic acid
Catalog No.:BCN8547
CAS No.:85031-59-0
- Acetyl Perisesaccharide C
Catalog No.:BCN8666
CAS No.:110764-09-5
- Z-VDVAD-FMK
Catalog No.:BCC1138
CAS No.:N/A
- Isoboonein
Catalog No.:BCN4545
CAS No.:99946-04-0
- Bullatantriol
Catalog No.:BCN4543
CAS No.:99933-32-1
- 3-(3,4-Dihydroxyphenyl)-1-n-propylpyrrolidine hydrobromide
Catalog No.:BCN8535
CAS No.:99933-30-9
- RGD (Arg-Gly-Asp) Peptides
Catalog No.:BCC5349
CAS No.:99896-85-2
- Panasenoside
Catalog No.:BCN8554
CAS No.:31512-06-8
- Ginsenoside Ra2
Catalog No.:BCN8555
CAS No.:83459-42-1
- Uvarigranol B
Catalog No.:BCN8556
CAS No.:164204-79-9
- Rupestonic acid
Catalog No.:BCN8557
CAS No.:115473-63-7
- Dihydropalmatine
Catalog No.:BCN8558
CAS No.:26067-60-7
- Anemarrhenasaponin I
Catalog No.:BCN8559
CAS No.:163047-21-0
- Glabrolide
Catalog No.:BCN8560
CAS No.:10401-33-9
- Acanthopanaxoside B
Catalog No.:BCN8561
CAS No.:915792-03-9
- Erigoster B
Catalog No.:BCN8562
CAS No.:849777-61-3
- 3'-Demethylnobiletin
Catalog No.:BCN8563
CAS No.:112448-39-2
- Lancifodilactone F
Catalog No.:BCN8564
CAS No.:850878-47-6
- Periplocoside N
Catalog No.:BCN8565
CAS No.:39946-41-3
Trans-sodium crocetinate provides neuroprotection against cerebral ischemia and reperfusion in obese mice.[Pubmed:25491171]
J Neurosci Res. 2015 Apr;93(4):615-22.
Trans-sodium crocetinate (TSC) is a novel synthetic carotenoid compound that improves diffusion of small molecules, including oxygen, in solutions. TSC provides neuroprotection in healthy rats and rabbits. This study seeks to determine whether TSC is neuroprotective in obese mice. Sixteen-week-old CD-1 male mice that had been fed a high-fat diet for 10 weeks were subjected to a 90-min middle cerebral arterial occlusion (MCAO). They received TSC by two boluses through a tail vein 10 min after the onset of MCAO and reperfusion, respectively, with doses of 0.14, 0.28, and 0.7 mg/kg or by a bolus-infusion-bolus strategy with a dose of 0.14 mg/kg during MCAO. The neurological outcome was evaluated 72 hr after MCAO. Brain tissues were harvested 24 hr after MCAO to measure nitrotyrosine-containing proteins, 4-hydroxy-2-nonenal, matrix metalloproteinase (MMP)-2 and -9 activity and expression, and inflammatory cytokines. TSC given in the two-bolus strategy did not improve the neurological outcome. The bolus-infusion-bolus strategy significantly reduced brain edema, infarct volume, and hemorrhagic transformation and improved neurological functions. TSC reduced nitrotyrosine-containing proteins, MMP-9 activity and expression, and inflammatory cytokines in ischemic brain tissues. Our results indicate that TSC delivered by the bolus-infusion-bolus strategy provides neuroprotection in obese mice. This protection may occur through reduction of oxidative stress, MMP-9 activity, or inflammatory cytokines in the ischemic brain tissues.
Trans-sodium crocetinate improves outcomes in rodent models of occlusive and hemorrhagic stroke.[Pubmed:25128603]
Brain Res. 2014 Oct 2;1583:245-54.
Trans-sodium crocetinate (TSC) is a novel carotenoid compound capable of enhancing the diffusion of small molecules in aqueous solutions. TSC improves the diffusion of oxygen and glucose, and increases oxygenation in ischemic brain tissue. TSC also dampens the intensity of an ischemic challenge during an ongoing ischemic event. The current study examined the impact of TSC in rat models of ischemic and hemorrhagic stroke. Rat three vessel occlusion (3VO), and combined 3VO and one vessel occlusion (3VO/1VO) models of ischemic stroke were evaluated for structural and behavioral outcomes. The effects of TSC were also tested in a rat model of intracerebral hemorrhage (ICH). Delayed treatment with TSC reduced infarct volume in a rodent model of transient focal ischemia involving either 2 or 6h of ischemia. Neurological outcomes, based on a multi-scale assessment and automated gait analysis, also were improved by TSC treatment. Additionally, TSC reduced edema and hemorrhagic volume in a rat model of ICH. An optimal therapeutic candidate for early intervention in ischemic stroke should be effective when administered on a delayed basis and should not aggravate outcomes associated with hemorrhagic stroke. The current findings demonstrate that delayed TSC treatment improves outcomes in experimental models of both ischemic and hemorrhagic stroke. Together, these findings suggest that TSC may be a safe and beneficial therapeutic modality for early stroke intervention, irrespective of the type of stroke involved.
Trans sodium crocetinate with temozolomide and radiation therapy for glioblastoma multiforme.[Pubmed:27177177]
J Neurosurg. 2017 Feb;126(2):460-466.
OBJECTIVE A new drug, Trans sodium crocetinate (TSC), has been developed to enhance the delivery of oxygen to hypoxic tissues. Cancerous tumors, such as glioblastoma multiforme (GBM), are very hypoxic, and it has been suggested that radiation therapy (RT) is more beneficial if tumors are better oxygenated. A Phase I/II clinical trial was conducted to determine the effect of adding TSC to RT sessions. METHODS An open, single-arm clinical trial incorporating the standard of care (SOC) for GBM was conducted at 18 clinical sites. There were 6 weeks of RT consisting of 2 Gy/day for 5 days/week, beginning after an initial resection or stereotactic biopsy to confirm GBM. Temozolomide (TMZ), 75 mg/m(2), was given before each RT session. The TSC, 0.25 mg/kg, was intravenously administered around 45 minutes before an RT session 3 days/week, usually on Monday, Wednesday, and Friday. A Phase I run-in period included 2 cohorts. The first cohort contained 3 patients who were given a half dose of the intravenous TSC (that is, 0.25 mg/kg, 3 times per week for only the first 3 weeks of RT). After a Safety Monitoring Committee (SMC) had verified that no dose-limiting toxicity (DLT) had occurred, a second cohort of 6 patients was given the same dosage of TSC but for the full 6 weeks of RT. After the SMC verified that no DLTs had occurred, Phase II began, with the administration of the full 18 doses of TSC. Fifty additional patients were enrolled during Phase II. Following the completion of RT, the patients rested for a month. After that, SOC TMZ chemotherapy (150-200 mg/m(2)) was administered for 5 days of the 1st week of 6 monthly cycles. No TSC was administered during this chemotherapy phase or later in the trial. Any other follow-up therapies were administered at the discretion of the individual investigators. RESULTS Kaplan-Meier analysis showed that 36% of the full-dose TSC patients were alive at 2 years, compared with historical survival values ranging from 27% to 30% for the SOC. Survival for the biopsy-only subset of patients was 40%, as compared with 42.9% for those patients having a complete resection before treatment. In addition, 2 of the 3 Phase I, Cohort 1 patients survived at 2 years. Contrast MRI data suggested that considerable pseudoprogression had occurred. Both Karnofsky Performance Status (KPS) scores and quality of life (QOL) questionnaires indicated that a good quality of life existed for most patients throughout the trial. No serious adverse events occurring in the trial were attributed to TSC. CONCLUSIONS This trial contained a single arm consisting of 59 patients. The results strongly suggested that adding TSC during RT is beneficial for the treatment of GBM. Trans sodium crocetinate offers a novel, easily implemented way to combat hypoxia in tumor tissue. Clinical trial registration no.: NCT01465347 ( clinicaltrials.gov ).


