Ilexsaponin B2CAS# 108906-69-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108906-69-0 | SDF | Download SDF |
| PubChem ID | 134715184 | Appearance | Powder |
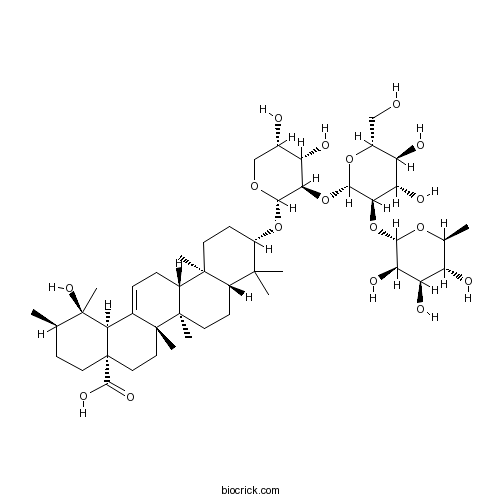
| Formula | C47H76O17 | M.Wt | 913.10 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2R,4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-[(2S,3R,4S,5S)-3-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-4,5-dihydroxyoxan-2-yl]oxy-1-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)OC6C(C(C(CO6)O)O)OC7C(C(C(C(O7)CO)O)O)OC8C(C(C(C(O8)C)O)O)O)C)C)C2C1(C)O)C)C(=O)O | ||
| Standard InChIKey | KWHRIYSRPSARCX-YNLCWPSQSA-N | ||
| Standard InChI | InChI=1S/C47H76O17/c1-21-11-16-47(41(56)57)18-17-44(6)23(37(47)46(21,8)58)9-10-27-43(5)14-13-28(42(3,4)26(43)12-15-45(27,44)7)62-39-35(30(51)24(49)20-59-39)64-40-36(33(54)31(52)25(19-48)61-40)63-38-34(55)32(53)29(50)22(2)60-38/h9,21-22,24-40,48-55,58H,10-20H2,1-8H3,(H,56,57)/t21-,22+,24+,25-,26+,27-,28+,29+,30+,31-,32-,33+,34-,35-,36-,37-,38+,39+,40+,43+,44-,45-,46-,47+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Ilexsaponin B2 is a cyclic nucleotide phosphodiesterase (PDE) inhibitor, it is active against PDEI and PDE5A dose-dependently in vitro. |
| Targets | PDE | P-gp | cAMP |

Ilexsaponin B2 Dilution Calculator

Ilexsaponin B2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0952 mL | 5.4759 mL | 10.9517 mL | 21.9034 mL | 27.3793 mL |
| 5 mM | 0.219 mL | 1.0952 mL | 2.1903 mL | 4.3807 mL | 5.4759 mL |
| 10 mM | 0.1095 mL | 0.5476 mL | 1.0952 mL | 2.1903 mL | 2.7379 mL |
| 50 mM | 0.0219 mL | 0.1095 mL | 0.219 mL | 0.4381 mL | 0.5476 mL |
| 100 mM | 0.011 mL | 0.0548 mL | 0.1095 mL | 0.219 mL | 0.2738 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 28-Demethyl-beta-amyrone
Catalog No.:BCN8551
CAS No.:73493-60-4
- Vinaginsenoside R3
Catalog No.:BCN8550
CAS No.:156012-92-9
- Cistanoside F
Catalog No.:BCN8549
CAS No.:97411-47-7
- Rhein-8-glucoside
Catalog No.:BCN8548
CAS No.:34298-86-7
- Dihydroartemisinic acid
Catalog No.:BCN8547
CAS No.:85031-59-0
- Acetyl Perisesaccharide C
Catalog No.:BCN8666
CAS No.:110764-09-5
- Z-VDVAD-FMK
Catalog No.:BCC1138
CAS No.:N/A
- Isoboonein
Catalog No.:BCN4545
CAS No.:99946-04-0
- Bullatantriol
Catalog No.:BCN4543
CAS No.:99933-32-1
- 3-(3,4-Dihydroxyphenyl)-1-n-propylpyrrolidine hydrobromide
Catalog No.:BCN8535
CAS No.:99933-30-9
- RGD (Arg-Gly-Asp) Peptides
Catalog No.:BCC5349
CAS No.:99896-85-2
- Isoboonein acetate
Catalog No.:BCN4542
CAS No.:99891-77-7
- Trans sodium crocetinate
Catalog No.:BCN8553
CAS No.:591230-99-8
- Panasenoside
Catalog No.:BCN8554
CAS No.:31512-06-8
- Ginsenoside Ra2
Catalog No.:BCN8555
CAS No.:83459-42-1
- Uvarigranol B
Catalog No.:BCN8556
CAS No.:164204-79-9
- Rupestonic acid
Catalog No.:BCN8557
CAS No.:115473-63-7
- Dihydropalmatine
Catalog No.:BCN8558
CAS No.:26067-60-7
- Anemarrhenasaponin I
Catalog No.:BCN8559
CAS No.:163047-21-0
- Glabrolide
Catalog No.:BCN8560
CAS No.:10401-33-9
- Acanthopanaxoside B
Catalog No.:BCN8561
CAS No.:915792-03-9
- Erigoster B
Catalog No.:BCN8562
CAS No.:849777-61-3
- 3'-Demethylnobiletin
Catalog No.:BCN8563
CAS No.:112448-39-2
- Lancifodilactone F
Catalog No.:BCN8564
CAS No.:850878-47-6
Rapid Screening of Potential Phosphodiesterase Inhibitors from the Roots of Ilex pubescens Hook. et Arn. Using a Combination of Ultrafiltration and LC-MS.[Pubmed:28424739]
Evid Based Complement Alternat Med. 2017;2017:2749643.
The cyclic nucleotide phosphodiesterase (PDE) plays an important role in regulating the levels of second messenger molecules cAMP and cGMP. Various PDE inhibitors have been successfully developed into drugs for targeted diseases. In addition, PDE inhibitors can also be found in different foods and natural medicines. In this study, ultrafiltration liquid chromatography-diode-array detector-electrospray ionization-ion-trap-time-of-flight-mass spectrometry (ultrafiltration LC-DAD-ESI-IT-TOF-MS) was applied to screen PDE inhibitors from the roots of Ilex pubescens Hook. et Arn. As a result, 11 major compounds were identified in I. pubescens roots, with nine compounds as potential PDE inhibitors, among which five were further confirmed to be active against PDEI and PDE5A dose-dependently in vitro, with ilexsaponin A1 and Ilexsaponin B2 being the strongest. HPLC quantification of these bioactive compounds suggested that they are major components in the plant. The results demonstrate that ultrafiltration LC-DAD-ESI-IT-TOF-MS is an efficient method for rapid screening of PDE inhibitors from natural medicines.
Study of Absorption Characteristics of the Total Saponins from Radix Ilicis Pubescentis in an In Situ Single-Pass Intestinal Perfusion (SPIP) Rat Model by Using Ultra Performance Liquid Chromatography (UPLC).[Pubmed:29104273]
Molecules. 2017 Nov 1;22(11). pii: molecules22111867.
In contrast to the extensively reported therapeutic activities, far less attention has been paid to the intestinal absorption of the total saponins from Radix Ilicis Pubescentis (in Chinese Mao-Dong-Qing, MDQ). This study aimed to investigate the intestinal absorption characteristics of ilexgenin A (C1), ilexsaponin A1 (C2), ilexsaponin B1 (C3), Ilexsaponin B2 (C4), ilexsaponin B3 (DC1), and ilexoside O (DC2) when administrated with the total saponins from MDQ (MDQ-TS). An UPLC method for simultaneous determination of C1, C2, C3, C4, DC1, and DC2 in intestinal outflow perfusate was developed and validated. The absorption characteristics of MDQ-TS were investigated by evaluating the effects of intestinal segments, drug concentration, P-glycoprotein (P-gp) inhibitor (verapomil), endocytosis inhibitor (amantadine) and ethylene diamine tetraacetic acid (EDTA, tight junction modulator) on the intestinal transportation of MDQ-TS by using a single-pass intestinal perfusion (SPIP) rat model, and the influence of co-existing components on the intestinal transport of the six saponins was discussed. The results showed that effective apparent permeability (Papp) of C1, C2, C3, C4, and DC2 administrated in MDQ-TS form had no segment-dependent changes at low and middle dosage levels. C1, C2, C3, D4, DC1, and DC2 administrated in MDQ-TS form all exhibited excellent transmembrane permeability with Papp > 0.12 x 10(-2) cm.min(-1). Meanwhile, Papp and effective absorption rate constant (Ka) values for the most saponins showed concentration dependence and saturation characteristics. After combining with P-gp inhibitor of verapamil, Papp of C2, C3, and DC1 in MDQ-TS group was significantly increased up to about 2.3-fold, 1.4-fold, and 3.4-fold, respectively in comparison to that of non-verapamil added group. Verapamil was found to improve the absorption of C2, C3, and DC1, indicating the involvement of an active transport mechanism in the absorption process. Compared with the non-amantadine added group, the absorption of C1, C2, C4, DC1, and DC2 were decreased by 40%, 71%, 31%, 53%, and 100%, respectively. Papp for the six target compounds increased up to about 1.2-2.1-fold in comparison with the non-EDTA added, respectively. The gastrointestinal transport of MDQ-TS could be greatly promoted by EDTA, and inhibited by amantadine, implying that the intestinal absorption of MDQ-TS was by passive diffusion and endocytosis process. Compared with monomer administration group, the intestinal absorption of C3, C4, DC1, and DC2 was significantly improved by co-existing components in MDQ-TS, and the non-absorbable saponins of C4, DC1, and DC2 unexpectedly showed sufficient intestinal permeability with Papp > 0.12 x 10(-2) cm.min(-1). This suggested that compounds orally administrated in TCM extract forms displayed unique intestinal absorption characteristics different from those of monomers, and the enhancing intestinal absorption of MDQ-TS reflected a holistic and specific view of traditional Chinese medicines (TCMs).


