BullatantriolCAS# 99933-32-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99933-32-1 | SDF | Download SDF |
| PubChem ID | 71430886 | Appearance | Cryst. |
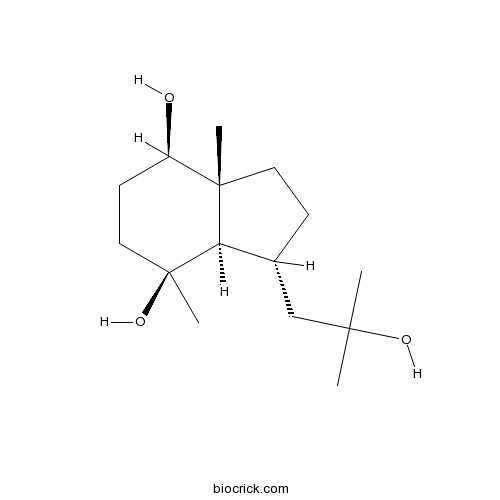
| Formula | C15H28O3 | M.Wt | 256.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3R,3aR,4S,7R,7aR)-3-(2-hydroxy-2-methylpropyl)-4,7a-dimethyl-2,3,3a,5,6,7-hexahydro-1H-indene-4,7-diol | ||
| SMILES | CC12CCC(C1C(CCC2O)(C)O)CC(C)(C)O | ||
| Standard InChIKey | JQHTXZNYHSCIFE-FPVZYODXSA-N | ||
| Standard InChI | InChI=1S/C15H28O3/c1-13(2,17)9-10-5-7-14(3)11(16)6-8-15(4,18)12(10)14/h10-12,16-18H,5-9H2,1-4H3/t10-,11-,12-,14+,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Bullatantriol has a stimulative effect on significantly proliferation and differentiation of culture osteoblasts. 2. Bullatantriol may have weak antibacterial activities. |
| Targets | Antifection |

Bullatantriol Dilution Calculator

Bullatantriol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9002 mL | 19.5008 mL | 39.0016 mL | 78.0031 mL | 97.5039 mL |
| 5 mM | 0.78 mL | 3.9002 mL | 7.8003 mL | 15.6006 mL | 19.5008 mL |
| 10 mM | 0.39 mL | 1.9501 mL | 3.9002 mL | 7.8003 mL | 9.7504 mL |
| 50 mM | 0.078 mL | 0.39 mL | 0.78 mL | 1.5601 mL | 1.9501 mL |
| 100 mM | 0.039 mL | 0.195 mL | 0.39 mL | 0.78 mL | 0.975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-(3,4-Dihydroxyphenyl)-1-n-propylpyrrolidine hydrobromide
Catalog No.:BCN8535
CAS No.:99933-30-9
- RGD (Arg-Gly-Asp) Peptides
Catalog No.:BCC5349
CAS No.:99896-85-2
- Isoboonein acetate
Catalog No.:BCN4542
CAS No.:99891-77-7
- Kaempferol 3-O-arabinoside
Catalog No.:BCN4541
CAS No.:99882-10-7
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
- Rotigotine
Catalog No.:BCC1907
CAS No.:99755-59-6
- Scholaricine
Catalog No.:BCN4539
CAS No.:99694-90-3
- 14-Benzoylneoline
Catalog No.:BCN6493
CAS No.:99633-05-3
- Ro 19-4603
Catalog No.:BCC7228
CAS No.:99632-94-7
- Uncinatone
Catalog No.:BCN4547
CAS No.:99624-92-7
- ent-3beta,18-Dihydroxylabda-8(17),13E-dien-15-oic acid
Catalog No.:BCN7669
CAS No.:99624-39-2
- Kazinol A
Catalog No.:BCN3388
CAS No.:99624-28-9
- Isoboonein
Catalog No.:BCN4545
CAS No.:99946-04-0
- Z-VDVAD-FMK
Catalog No.:BCC1138
CAS No.:N/A
- Acetyl Perisesaccharide C
Catalog No.:BCN8666
CAS No.:110764-09-5
- Dihydroartemisinic acid
Catalog No.:BCN8547
CAS No.:85031-59-0
- Rhein-8-glucoside
Catalog No.:BCN8548
CAS No.:34298-86-7
- Cistanoside F
Catalog No.:BCN8549
CAS No.:97411-47-7
- Vinaginsenoside R3
Catalog No.:BCN8550
CAS No.:156012-92-9
- 28-Demethyl-beta-amyrone
Catalog No.:BCN8551
CAS No.:73493-60-4
- Ilexsaponin B2
Catalog No.:BCN8552
CAS No.:108906-69-0
- Trans sodium crocetinate
Catalog No.:BCN8553
CAS No.:591230-99-8
- Panasenoside
Catalog No.:BCN8554
CAS No.:31512-06-8
- Ginsenoside Ra2
Catalog No.:BCN8555
CAS No.:83459-42-1
Sesquiterpenoids from Homalomena occulta affect osteoblast proliferation, differentiation and mineralization in vitro.[Pubmed:18649899]
Phytochemistry. 2008 Sep;69(12):2367-73.
Chemical investigation of rhizomes of Homalomena occulta (Lours) resulted in isolation and identification of two sesquiterpenoids (6,7), and one daucane ester 8, together with five known sesquiterpenoids, oplodiol, oplopanone, homalomenol C, Bullatantriol, and 1beta,4beta,7alpha-trihydroxyeudesmane. Their structures were elucidated using 1D and 2D NMR spectroscopic and X-ray analyses. The chloroform extract of this plant and compounds 1-7 were tested in vitro for their activities in stimulating osteoblast (OB) proliferation, differentiation and mineralization. Compounds 1-4 had a stimulative effect on significantly proliferation and differentiation of culture osteoblasts, while the chloroform extract and 1 significantly stimulated mineralization of cultured osteoblasts in vitro.
Sesquiterpenoids from the rhizomes of Homalomena occulta.[Pubmed:22573367]
Planta Med. 2012 Jun;78(10):1010-4.
Phytochemical investigation on the rhizomes of Homalomena occulta resulted in the isolation of five new sesquiterpenoids, namely cadinane-4beta,5alpha,10alpha-triol (1), 5(11)-epoxycadinane-4beta,5beta,10beta,11-tetraol (2), bullatantiol-1beta-methyl malate (3), 1beta,4beta,7alpha-trihydroxyeudesmane-1beta-methyl malate (6), and 1beta,4alpha,7-trihydroxyeudes-mane (7), together with five known sesquiterpenoids, Bullatantriol (4), acetylBullatantriol (5), 1beta,4beta,7alpha-trihydroxyeudesmane (8), 1beta,4beta,7beta-trihydroxyeude-smane (9), and pterodontriol (10). Their structures were elucidated on the basis of spectroscopic evidences, including various 1D and 2D NMR and HR-ESI-MS. The structure of 1 was further confirmed by single-crystal X-ray diffraction analysis.
[Chemical constituents of Acorus calamus].[Pubmed:23373216]
Zhongguo Zhong Yao Za Zhi. 2012 Nov;37(22):3430-3.
OBJECTIVE: To study the chemical constituents contained in Acorus calamus. METHOD: The chemical constituents were separated and purified by various chromatographic methods including silica gel, ODS, HPLC and Sephadex LH-20, and their structures were identified on the basis of analysis on spectroscopic data. RESULT: Ten compounds were separated from A. calamus and identified as 1beta, 4beta, 7alpha-trihydroxyeudesmane (1), Bullatantriol (2), teuclatriol (3), threo-1', 2'-dihydroxyasarone (4), erythro-1', 2'-dihydroxyasarone (5), (+)-de-4'-O-methyleudesmin (6), (+)-de-4'-0-methylmagnolin (7), (+)-eudesmin (8), (+)-magnolin (9) and beta-sitosterol (10), respectively. CONCLUSION: Compounds 1-2,4-9 were separated from this plant for the first time. Specifically, compounds 1-2,6-9 were obtained from Acorus genus for the first time.
Three new sesquiterpenoids from the aerial parts of Homalomena occulta.[Pubmed:17511005]
Chem Biodivers. 2007 May;4(5):925-31.
Three new eudesmane-type sesquiterpenoids, compounds 1-3, and eight known constituents, including mucrolidin (4), 1beta,4beta,7alpha-trihydroxyeudesmane (5), 1beta,4beta,6beta,11-tetrahydroxyeudesmane (6), oplodiol (7), Bullatantriol (8), acetylBullatantriol (9), homalomenol (10), and maristeminol (11), were isolated from the aerial parts of Homalomena occulta. Their structures were determined by interpretation of spectroscopic and mass-spectrometric data, and their antimicrobial activities toward six different bacterial strains were tested. Most of the compounds showed weak antibacterial activities in an agar-diffusion assay.


