Ro 19-4603Benzodiazepine inverse agonist CAS# 99632-94-7 |
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- PCI-32765 (Ibrutinib)
Catalog No.:BCC1266
CAS No.:936563-96-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99632-94-7 | SDF | Download SDF |
| PubChem ID | 127382 | Appearance | Powder |
| Formula | C15H17N3O3S | M.Wt | 319.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
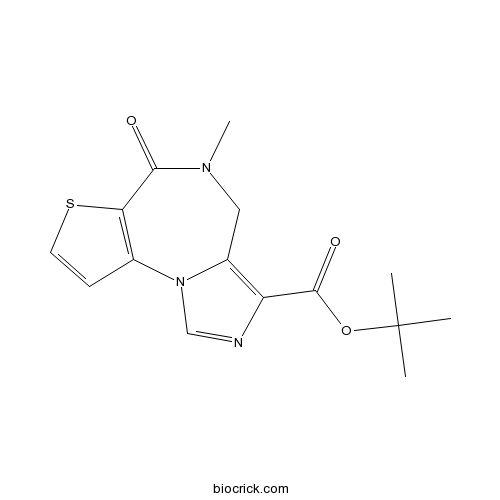
| SMILES | CC(C)(C)OC(=O)C1=C2CN(C(=O)C3=C(N2C=N1)C=CS3)C | ||
| Standard InChIKey | ZIGMMUKDYCABPW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H17N3O3S/c1-15(2,3)21-14(20)11-10-7-17(4)13(19)12-9(5-6-22-12)18(10)8-16-11/h5-6,8H,7H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Benzodiazepine inverse agonist. Binds with high affinity to both diazepam-sensitive (DS) and diazepam-insensitive (DI) GABAA receptors (Ki values are ~ 0.2 and ~ 2.6 nM for DS and DI receptors respectively). Antagonizes effects of ethanol on locomotor behavior and suppresses ethanol intake in alcohol-preferring rats. |

Ro 19-4603 Dilution Calculator

Ro 19-4603 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1311 mL | 15.6553 mL | 31.3107 mL | 62.6213 mL | 78.2767 mL |
| 5 mM | 0.6262 mL | 3.1311 mL | 6.2621 mL | 12.5243 mL | 15.6553 mL |
| 10 mM | 0.3131 mL | 1.5655 mL | 3.1311 mL | 6.2621 mL | 7.8277 mL |
| 50 mM | 0.0626 mL | 0.3131 mL | 0.6262 mL | 1.2524 mL | 1.5655 mL |
| 100 mM | 0.0313 mL | 0.1566 mL | 0.3131 mL | 0.6262 mL | 0.7828 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Uncinatone
Catalog No.:BCN4547
CAS No.:99624-92-7
- ent-3beta,18-Dihydroxylabda-8(17),13E-dien-15-oic acid
Catalog No.:BCN7669
CAS No.:99624-39-2
- Kazinol A
Catalog No.:BCN3388
CAS No.:99624-28-9
- Kazinol B
Catalog No.:BCN4538
CAS No.:99624-27-8
- Isothymonin
Catalog No.:BCN3393
CAS No.:99615-01-7
- Leucanthogenin
Catalog No.:BCN7932
CAS No.:99615-00-6
- Ondansetron
Catalog No.:BCC5043
CAS No.:99614-02-5
- Ondansetron HCl
Catalog No.:BCC2493
CAS No.:99614-01-4
- Sertaconazole nitrate
Catalog No.:BCC4716
CAS No.:99592-39-9
- Sertaconazole
Catalog No.:BCC9146
CAS No.:99592-32-2
- Neuropeptide FF
Catalog No.:BCC5983
CAS No.:99566-27-5
- K 252a
Catalog No.:BCC7152
CAS No.:99533-80-9
- 14-Benzoylneoline
Catalog No.:BCN6493
CAS No.:99633-05-3
- Scholaricine
Catalog No.:BCN4539
CAS No.:99694-90-3
- Rotigotine
Catalog No.:BCC1907
CAS No.:99755-59-6
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
- Kaempferol 3-O-arabinoside
Catalog No.:BCN4541
CAS No.:99882-10-7
- Isoboonein acetate
Catalog No.:BCN4542
CAS No.:99891-77-7
- RGD (Arg-Gly-Asp) Peptides
Catalog No.:BCC5349
CAS No.:99896-85-2
- 3-(3,4-Dihydroxyphenyl)-1-n-propylpyrrolidine hydrobromide
Catalog No.:BCN8535
CAS No.:99933-30-9
- Bullatantriol
Catalog No.:BCN4543
CAS No.:99933-32-1
- Isoboonein
Catalog No.:BCN4545
CAS No.:99946-04-0
- Z-VDVAD-FMK
Catalog No.:BCC1138
CAS No.:N/A
- Acetyl Perisesaccharide C
Catalog No.:BCN8666
CAS No.:110764-09-5
Different functional effect of Ro 15-4513 and Ro 19-4603 on synaptic responses of Purkinje cells in the rat cerebellar slice.[Pubmed:7895057]
Brain Res. 1994 Dec 5;665(2):222-8.
Modulation of GABA-mediated neurotransmissions by Ro 15-4513 in cerebellar slices was assessed following stimulation of the parallel fibre input, which, in this preparation, preferentially activates the inhibitory interneurones innervating Purkinje cells. Peristimulus-time histogram analysis of inhibitory responses of spontaneously-active Purkinje cells showed only a decrease in the duration of inhibition induced by Ro 19-4603. This is consistent with inverse agonism on the BZ1 receptors associated with postsynaptic GABAA receptors on Purkinje cells. 1 microM Ro 15-4513 induced a similar response but 100 nM Ro 15-4513 induced a biphasic response, with an increase in duration of inhibition preceding the decrease during continued perfusion of the compound. At lower concentrations of Ro 15-4513 the increase in inhibition predominated, the minimal effective concentration being 10 pM. 1 microM flumazenil blocked both components of this response to 100 nM Ro 15-4513, but at 100 nM flumazenil only blocked the decrease in inhibition. The ability of Ro 15-4513 but not Ro 19-4603 to enhance inhibition and its relative insensitivity to 100 nM flumazenil, parallel the affinities of these compounds for diazepam-insensitive (DI) binding sites in the cerebellum. These data suggest that the enhancement of inhibition induced by Ro 15-4513 results from its inverse agonist activity on DI receptors causing disinhibition of both granule cells and their parallel fibres and increased sensitivity to the electrical stimuli inducing activation of the inhibitory interneurones innervating Purkinje cells.
Ro 19-4603, a benzodiazepine receptor inverse agonist, attenuates voluntary ethanol consumption in rats selectively bred for high ethanol preference.[Pubmed:1965120]
Alcohol Alcohol. 1990;25(5):449-52.
The effect of Ro 19-4603, a novel potent partial inverse agonist of benzodiazepine (BZ) receptors, on voluntary ethanol intake was examined in a rat line selectively bred for ethanol preference (Sardinian ethanol preferring, sP, rats). Ro 19-4603, 1 mg/kg i.p., three times daily, reduced voluntary ethanol consumption by about 40% during 7 days of treatment, but failed to reduce water intake. The results suggest that the GABA/BZ receptor complex may play a role in the reinforcing property of ethanol.
Convulsant action of a benzodiazepine receptor agonist/inverse agonist Ro 19-4603 in developing rats.[Pubmed:7845475]
Naunyn Schmiedebergs Arch Pharmacol. 1994 Oct;350(4):393-7.
An inverse benzodiazepine receptor agonist Ro 19-4603, administered intraperitoneally, was found to induce two types of motor seizures, i.e. minimal, predominantly clonic and major, generalized tonic-clonic, in rats at all developmental stages studied (7, 12, 18 and 25 days old). The developmental profile of the two types of seizure was different. Minimal seizures could be induced easily in the two youngest groups, whereas there were no marked differences in the induction of major seizures between the age groups. A lethal outcome was more common in 18- and 25-day-old rats than in younger animals. The convulsant action of the benzodiazepine agonist/inverse agonist Ro 19-4603 shows only quantitative changes during post-natal development in the rat.
Anxiogenic effects of a benzodiazepine receptor partial inverse agonist, RO 19-4603, in a light/dark choice situation.[Pubmed:2165618]
Pharmacol Biochem Behav. 1990 Jul;36(3):593-6.
In a light/dark choice procedure, the imidazothienodiazepinone Ro 19-4603, given alone, induced a dose-dependent decrease in the time spent by mice in the lit box as well in the number of transitions between the two boxes. These data confirm the anxiogenic intrinsic properties of inverse agonists of the benzodiazepine receptor. Since Ro 19-4603 also reversed the anxiolytic effects of ethanol and exhibited proconvulsant properties, it is suggested that the antagonistic action of this drug against ethanol could be due to an additive rather than an interactive process.
The novel benzodiazepine inverse agonist RO19-4603 antagonizes ethanol motivated behaviors: neuropharmacological studies.[Pubmed:9518641]
Brain Res. 1998 Feb 16;784(1-2):256-75.
The novel imidazothienodiazepine inverse agonist RO19-4603 has been reported to attenuate EtOH intake in home cage drinking tests for at least 24 h post-drug administration after systemic administration. In the present study, selectively bred alcohol-preferring (P) rats were trained under a concurrent (FR4-FR4) operant schedule to press one lever for EtOH (10% v/v) and another lever for saccharin (0.05% or 0.75% g/v), then dose-response and timecourse effects of RO19-4603 were evaluated. Systemic RO19-4603 injections (0.0045-0.3 mg/kg; i.p.) profoundly reduced EtOH responding by as much as 97% of vehicle control on day 1. No effects were seen on saccharin responding except with the highest dose level (0.3 mg/kg). In a second experiment, microinjections of RO19-4603 (2-100 ng) directly into the nucleus accumbens (NA) suppressed EtOH responding on day 1 by as much as 53% of control: Control injections dorsal to the NA or ventral tegmental area did not significantly alter EtOH or saccharin responding. On day 2, rats in both experiments received no RO19-4603 treatments; however, all 7 of the i.p. doses, and all 3 of the intra-NA infusions continued to significantly suppress EtOH responding by 43-85% of vehicle control levels. In addition, i.p. injections of RO19-4603 produced a dose-dependent decrease in the slope of the cumulative record for EtOH responding, while concomitantly producing a dose-dependent increase in the slope for saccharin responding. RO19-4603's actions appear to be mediated via recognition sites at GABAA-BDZ receptors which regulate EtOH reinforcement, and not via mechanisms regulating ingestive behaviors. Based on recent in situ hybridization studies in our laboratory, we hypothesize that occupation of alpha4 containing GABAA diazepam insensitive (DI) receptors in the NA, may mediate in part, the RO19-4603 suppression of EtOH responding in EtOH-seeking P rats.
Effects of the benzodiazepine inverse agonist RO19-4603 on the maintenance of tolerance to a single dose of ethanol.[Pubmed:7562476]
J Pharmacol Exp Ther. 1995 Sep;274(3):1105-12.
The time course of the novel benzodiazepine inverse agonist, RO19--4603 (0.075 or 0.150 mg/kg) in antagonizing the depressant effects of ethanol (EtOH) (0.50, 1.0 and 1.5 g/kg) and the development of tolerance on locomotor behaviors (e.g., ambulatory count, total distance and stereotypy count) were investigated in Sprague-Dawley rats given EtOH injections spaced at 24-hr intervals. A single dose of RO19--4603 prevented the development of tolerance to the 0.50- and 1.0-g/kg EtOH doses 24-hr post-RO19--4603 administration on most locomotor behaviors. On Day 1, the 0.150-mg/kg RO19--4603 dose prevented the reduction of motor behaviors after the 1.0- and 1.5-g/kg EtOH doses, whereas the 0.075-mg/kg RO19--4603 dose prevented the reduction of motor behaviors only after the 1.5-g/kg EtOH dose. The 0.075- and 0.150-mg/kg RO19--4603 doses also prevented the EtOH-induced reduction of motor behaviors after the 1.5-g/kg EtOH dose 24-hr post-RO19--4603 administration. RO19--4603 was without effect on activity when given alone. These data suggest that the motor impairing effects of EtOH and the development of tolerance to them may involve gamma-aminobutyric acidA-benzodiazepine receptor mechanisms that when occupied, even briefly by certain benzodiazepine inverse agonists, produce long-lasting effects on locomotion and tolerance.
High affinity ligands for 'diazepam-insensitive' benzodiazepine receptors.[Pubmed:1311690]
Eur J Pharmacol. 1992 Jan 14;225(1):63-8.
Structurally diverse compounds have been shown to possess high affinities for benzodiazepine receptors in their 'diazepam-sensitive' (DS) conformations. In contrast, only the imidazobenzodiazepinone Ro 15-4513 has been shown to exhibit a high affinity for the 'diazepam-insensitive' (DI) conformation of benzodiazepine receptors. We examined a series of 1,4-diazepines containing one or more annelated ring systems for their affinities at DI and DS benzodiazepine receptors, several 1,4-diazepinone carboxylates including Ro 19-4603, Ro 16-6028 and Ro 15-3505 were found to possess high affinities (Ki approximately 2.6-20 nM) for DI. Nonetheless, among the ligands examined, Ro 15-4513 was the only substance with a DI/DS potency ratio approximately 1; other substances had ratios ranging from 13 to greater than 1000. Ligands with high to moderate affinities at DI were previously classified as partial agonists, antagonists, or partial inverse agonists at DS benzodiazepine receptors, but behaved as 'GABA neutral' (antagonist) substances at DI. The identification of several additional high affinity ligands at DI benzodiazepine receptors may be helpful in elucidating the pharmacological and physiological importance of these sites.


