IsothymoninCAS# 99615-01-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99615-01-7 | SDF | Download SDF |
| PubChem ID | 11726019 | Appearance | Yellow cryst. |
| Formula | C18H16O8 | M.Wt | 360.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
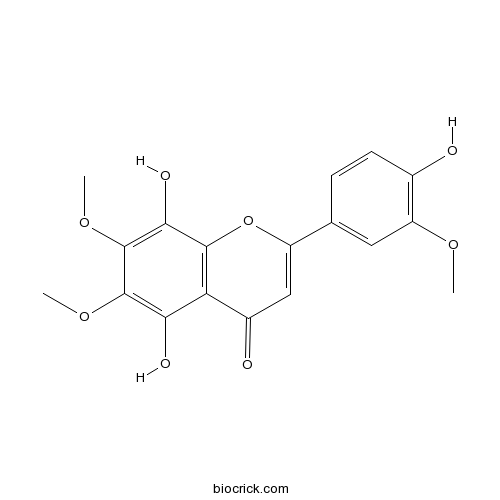
| Chemical Name | 5,8-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-6,7-dimethoxychromen-4-one | ||
| SMILES | COC1=C(C=CC(=C1)C2=CC(=O)C3=C(O2)C(=C(C(=C3O)OC)OC)O)O | ||
| Standard InChIKey | CSDGLNFYKPCMSZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O8/c1-23-12-6-8(4-5-9(12)19)11-7-10(20)13-14(21)17(24-2)18(25-3)15(22)16(13)26-11/h4-7,19,21-22H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isothymonin has antioxidant activity. 2. Isothymonin displays cyclooxygenase-1 inhibitory activity. |
| Targets | COX |

Isothymonin Dilution Calculator

Isothymonin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7755 mL | 13.8773 mL | 27.7546 mL | 55.5093 mL | 69.3866 mL |
| 5 mM | 0.5551 mL | 2.7755 mL | 5.5509 mL | 11.1019 mL | 13.8773 mL |
| 10 mM | 0.2775 mL | 1.3877 mL | 2.7755 mL | 5.5509 mL | 6.9387 mL |
| 50 mM | 0.0555 mL | 0.2775 mL | 0.5551 mL | 1.1102 mL | 1.3877 mL |
| 100 mM | 0.0278 mL | 0.1388 mL | 0.2775 mL | 0.5551 mL | 0.6939 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Leucanthogenin
Catalog No.:BCN7932
CAS No.:99615-00-6
- Ondansetron
Catalog No.:BCC5043
CAS No.:99614-02-5
- Ondansetron HCl
Catalog No.:BCC2493
CAS No.:99614-01-4
- Sertaconazole nitrate
Catalog No.:BCC4716
CAS No.:99592-39-9
- Sertaconazole
Catalog No.:BCC9146
CAS No.:99592-32-2
- Neuropeptide FF
Catalog No.:BCC5983
CAS No.:99566-27-5
- K 252a
Catalog No.:BCC7152
CAS No.:99533-80-9
- L-651,582
Catalog No.:BCC7561
CAS No.:99519-84-3
- 3-Ethoxy-4-ethoxycarbonyl phenylacetic acid
Catalog No.:BCC8629
CAS No.:99469-99-5
- 1-Chloroethyl cyclohexyl carbonate
Catalog No.:BCC8463
CAS No.:99464-83-2
- Ampiroxicam
Catalog No.:BCC4426
CAS No.:99464-64-9
- BTZO 1
Catalog No.:BCC7886
CAS No.:99420-15-2
- Kazinol B
Catalog No.:BCN4538
CAS No.:99624-27-8
- Kazinol A
Catalog No.:BCN3388
CAS No.:99624-28-9
- ent-3beta,18-Dihydroxylabda-8(17),13E-dien-15-oic acid
Catalog No.:BCN7669
CAS No.:99624-39-2
- Uncinatone
Catalog No.:BCN4547
CAS No.:99624-92-7
- Ro 19-4603
Catalog No.:BCC7228
CAS No.:99632-94-7
- 14-Benzoylneoline
Catalog No.:BCN6493
CAS No.:99633-05-3
- Scholaricine
Catalog No.:BCN4539
CAS No.:99694-90-3
- Rotigotine
Catalog No.:BCC1907
CAS No.:99755-59-6
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
- Kaempferol 3-O-arabinoside
Catalog No.:BCN4541
CAS No.:99882-10-7
- Isoboonein acetate
Catalog No.:BCN4542
CAS No.:99891-77-7
- RGD (Arg-Gly-Asp) Peptides
Catalog No.:BCC5349
CAS No.:99896-85-2
Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn.[Pubmed:10782484]
Phytomedicine. 2000 Mar;7(1):7-13.
Anti-oxidant bioassay-directed extraction of the fresh leaves and stems of Ocimum sanctum and purification of the extract yielded the following compounds; cirsilineol [1], cirsimaritin [2], isothymusin [3], Isothymonin [4], apigenin [5], rosmarinic acid [6], and appreciable quantities of eugenol. The structures of compounds 1-6 were established using spectroscopic methods. Compounds 1 and 5 were isolated previously from O. sanctum whereas compounds 2 and 3 are here identified for the first time from O. sanctum. Eugenol, a major component of the volatile oil, and compounds 1, 3, 4, and 6 demonstrated good antioxidant activity at 10-microM concentrations. Anti-inflammatory activity or cyclooxygenase inhibitory activity of these compounds were observed. Eugenol demonstrated 97% cyclooxygenase-1 inhibitory activity when assayed at 1000-microM concentrations. Compounds 1, 2, and 4-6 displayed 37, 50, 37, 65, and 58% cyclooxygenase-1 inhibitory activity, respectively, when assayed at 1000-microM concentrations. Eugenol and compounds 1, 2, 5, and 6 demonstrated cyclooxygenase-2 inhibitory activity at slightly higher levels when assayed at 1000-microM concentrations. The activities of compounds 1-6 were comparable to ibuprofen, naproxen, and aspirin at 10-, 10-, and 1000-microM concentrations, respectively. These results support traditional uses of O. sanctum and identify the compounds responsible.


