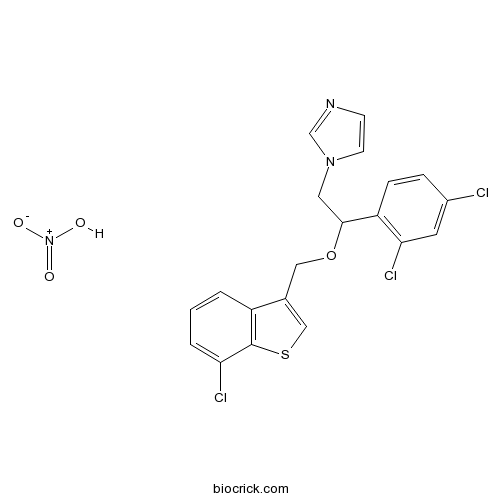
Sertaconazole nitrateBroad-spectrum antifungal CAS# 99592-39-9 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99592-39-9 | SDF | Download SDF |
| PubChem ID | 200103 | Appearance | Powder |
| Formula | C20H16Cl3N3O4S | M.Wt | 500.78 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (199.69 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1-[2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole;nitric acid | ||
| SMILES | C1=CC2=C(C(=C1)Cl)SC=C2COC(CN3C=CN=C3)C4=C(C=C(C=C4)Cl)Cl.[N+](=O)(O)[O-] | ||
| Standard InChIKey | HAAITRDZHUANGT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H15Cl3N2OS.HNO3/c21-14-4-5-16(18(23)8-14)19(9-25-7-6-24-12-25)26-10-13-11-27-20-15(13)2-1-3-17(20)22;2-1(3)4/h1-8,11-12,19H,9-10H2;(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sertaconazole nitrate Dilution Calculator

Sertaconazole nitrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9969 mL | 9.9844 mL | 19.9688 mL | 39.9377 mL | 49.9221 mL |
| 5 mM | 0.3994 mL | 1.9969 mL | 3.9938 mL | 7.9875 mL | 9.9844 mL |
| 10 mM | 0.1997 mL | 0.9984 mL | 1.9969 mL | 3.9938 mL | 4.9922 mL |
| 50 mM | 0.0399 mL | 0.1997 mL | 0.3994 mL | 0.7988 mL | 0.9984 mL |
| 100 mM | 0.02 mL | 0.0998 mL | 0.1997 mL | 0.3994 mL | 0.4992 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sertaconazole nitrate is a topical broad-spectrum antifungal that is developed to provide an additional agent for the treatment of superficial cutaneous and mucosal infections.
- Sertaconazole
Catalog No.:BCC9146
CAS No.:99592-32-2
- Neuropeptide FF
Catalog No.:BCC5983
CAS No.:99566-27-5
- K 252a
Catalog No.:BCC7152
CAS No.:99533-80-9
- L-651,582
Catalog No.:BCC7561
CAS No.:99519-84-3
- 3-Ethoxy-4-ethoxycarbonyl phenylacetic acid
Catalog No.:BCC8629
CAS No.:99469-99-5
- 1-Chloroethyl cyclohexyl carbonate
Catalog No.:BCC8463
CAS No.:99464-83-2
- Ampiroxicam
Catalog No.:BCC4426
CAS No.:99464-64-9
- BTZO 1
Catalog No.:BCC7886
CAS No.:99420-15-2
- Methyl rosmarinate
Catalog No.:BCN4536
CAS No.:99353-00-1
- Kadsurin A
Catalog No.:BCN6515
CAS No.:99340-07-5
- Venlafaxine Hydrochloride
Catalog No.:BCC2513
CAS No.:99300-78-4
- Levodropropizine
Catalog No.:BCC4520
CAS No.:99291-25-5
- Ondansetron HCl
Catalog No.:BCC2493
CAS No.:99614-01-4
- Ondansetron
Catalog No.:BCC5043
CAS No.:99614-02-5
- Leucanthogenin
Catalog No.:BCN7932
CAS No.:99615-00-6
- Isothymonin
Catalog No.:BCN3393
CAS No.:99615-01-7
- Kazinol B
Catalog No.:BCN4538
CAS No.:99624-27-8
- Kazinol A
Catalog No.:BCN3388
CAS No.:99624-28-9
- ent-3beta,18-Dihydroxylabda-8(17),13E-dien-15-oic acid
Catalog No.:BCN7669
CAS No.:99624-39-2
- Uncinatone
Catalog No.:BCN4547
CAS No.:99624-92-7
- Ro 19-4603
Catalog No.:BCC7228
CAS No.:99632-94-7
- 14-Benzoylneoline
Catalog No.:BCN6493
CAS No.:99633-05-3
- Scholaricine
Catalog No.:BCN4539
CAS No.:99694-90-3
- Rotigotine
Catalog No.:BCC1907
CAS No.:99755-59-6
Investigation of the Dermal Absorption and Irritation Potential of Sertaconazole Nitrate Anhydrous Gel.[Pubmed:27399763]
Pharmaceutics. 2016 Jul 7;8(3). pii: pharmaceutics8030021.
Effective topical therapy of cutaneous fungal diseases requires the delivery of the active agent to the target site in adequate concentrations to produce a pharmacological effect and inhibit the growth of the pathogen. In addition, it is important to determine the concentration of the drug in the skin in order to evaluate the subsequent efficacy and potential toxicity for topical formulations. For this purpose, an anhydrous gel containing Sertaconazole nitrate as a model drug was formulated and the amount of the drug in the skin was determined by in vitro tape stripping. The apparent diffusivity and partition coefficients were then calculated by a mathematical model describing the dermal absorption as passive diffusion through a pseudo-homogenous membrane. The skin irritation potential of the formulation was also assessed by using the in vitro Epiderm model. An estimation of the dermal absorption parameters allowed us to evaluate drug transport across the stratum corneum following topical application. The estimated concentration for the formulation was found to be higher than the MIC100 at the target site which suggested its potential efficacy for treating fungal infections. The skin irritation test showed the formulation to be non-irritating in nature. Thus, in vitro techniques can be used for laying the groundwork in developing efficient and non-toxic topical products.
Efficacy and Safety of Terbinafine Hydrochloride 1% Cream vs. Sertaconazole Nitrate 2% Cream in Tinea Corporis and Tinea Cruris: A Comparative Therapeutic Trial.[Pubmed:24249898]
Indian J Dermatol. 2013 Nov;58(6):457-60.
CONTEXT: To the best of our knowledge, till date no study comparing the efficacy and safety of terbinafine hydrochloride 1% cream and Sertaconazole nitrate 2% cream has been done in localized tinea corporis and tinea cruris. AIMS: This clinical trial was carried out to study and compare the efficacy of topical terbinafine hydrochloride 1% cream and Sertaconazole nitrate 2% cream in localized tinea corporis and tinea cruris and to know the adverse effects of these antifungal creams. SETTINGS AND DESIGN: In this prospective, single blind, randomized control trial with two arms, patient were randomized into two groups Group A (treatment with terbinafine cream) and Group B (treatment with sertaconazole cream). A total of 38 patients were enrolled for the study, 20 patients in group A and 18 patients in group B. But five patients of group A and three patients of group B were lost for follow-ups. Therefore sample size was of 30 patients with 15 patients in group A and group B each. MATERIALS AND METHODS: Patients in group A and B were treated with twice daily topical 1% terbinafine hydrochloride and 2% Sertaconazole nitrate cream respectively for a total duration of three weeks. Clinical improvement in signs and symptoms of each clinical parameter, namely itching, erythema, papules, pustules, vesicles, and scaling were graded weekly and clinical cure was assessed. KOH mount and culture was done weekly up to 3 weeks to access mycological cure. Fungal culture was done on Sabouraud's dextrose agar with chloramphenicol and cycloheximide. STATISTICAL ANALYSIS USED: Statistical analysis was done using students paired and unpaired t-tests from the data obtained. RESULTS: Comparison between Group A and Group B for complete cure (clinical and mycological) showed that at the end of 3 weeks both terbinafine and sertaconazole groups had 100% complete cure. When the two groups were compared for complete cure, at the end of 1(st) and 2(nd) week, statistically non-significant results were observed (P = 0.461 and P = 0.679 respectively). However, at the end of 2(nd) week, complete cure rate for terbinafine was 80% as compared to 73.35% for sertaconazole with no statistical significance. In both Group A and Group B, clinically significant local side effects like erythema, swelling, stinging sensation, or increased itching were not noticed. A majority of our patients in both the group showed Trichophyton rubrum followed by Trichophyton mentagrophytes growth on culture. In Group A, 11 patients showed growth of T. rubrum, 2 patients showed growth of T. mentagrophytes, and 1 patient had only KOH test positive. In Group B, 10 patients revealed growth of T. rubrum, followed by growth of T. mentagrophytes in 3 and Microsporum canis in 2 patients. The therapeutic response is more or less same in infection with different species. CONCLUSIONS: The newer fungistatic drug Sertaconazole nitrate 2% cream was as effective as terbinafine hydrochloride 1% cream which is one of the fungicidal drugs, though terbinafine hydrochloride 1% cream has higher rates of complete cure at the end of 2 weeks as compared to Sertaconazole nitrate 2% cream. Both the drugs showed good tolerability with no adverse effects.
Fabrication and Characterization of Sertaconazole Nitrate Microsponge as a Topical Drug Delivery System.[Pubmed:26997694]
Indian J Pharm Sci. 2015 Nov-Dec;77(6):675-80.
Present study was taken up to develop a topical formulation that releases the drug in controlled manner, reduce the side effects associated with topical drug delivery and improve product efficacy with aid of microsponges. Microsponges loaded with Sertaconazole nitrate were prepared by using quasi emulsion solvent diffusion with five different proportions of the polymer (Eudragit RS 100). The developed microsponges were analyzed for particle size, production yield, entrapment efficiency and drug content. Scanning electron microscopic images of microsponges revealed that they are spherical in shape and contain pores. Pore structure analysis was done by using mercury intrusion porosimetry technique, which confirmed the porous nature of microsponges. Microsponges were then incorporated in to a 1% corbopol gel and evaluated for pH, drug content, texture profile analysis and in vitro drug release. The batch F IV was found to be optimal as it shown 69.38% controlled drug release in 8 h that followed Higuchi model.
Design expert assisted formulation of topical bioadhesive gel of sertaconazole nitrate.[Pubmed:24511475]
Adv Pharm Bull. 2014;4(2):121-30.
PURPOSE: The objective of this work was to develop a bioadhesive topical gel of Sertaconazole nitrate with the help of response-surface approach. METHODS: Experiments were performed according to a 3-level factorial design to evaluate the effects of two independent variables [amount of Carbapol 934 = X1) and Sodium carboxymethylcellulose (NaCMC) = X2)] on the bioadhesive character of gel, rheological property of gel (consistency index), and in-vitro drug release. The best model was selected to fit the data. RESULTS: Mathematical equation was generated by Design Expert(R) software for the model which assists in determining the effect of independent variables. Response surface plots were also generated by the software for analyzing effect of the independent variables on the response. The effect of formulation variables on the product characteristics can be easily predicted and precisely interpreted by using a 3-level factorial design and generated quadratic mathematical equations. CONCLUSION: On the basis of product characteristics viscosity, bioadhesiveness, permeation study, in-vitro release, in-vivo studies, TPA and spreadability it can be concluded that the best batch of topical bioadhesive gel of Sertaconazole nitrate would be with 1% Carbopol 934 and 1% NaCMC.


