RGD (Arg-Gly-Asp) PeptidesInhibits integrin binding to RGD motifs CAS# 99896-85-2 |
- RG7388
Catalog No.:BCC1895
CAS No.:1229705-06-9
- MI-773
Catalog No.:BCC5155
CAS No.:1303607-07-9
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- MIRA-1
Catalog No.:BCC2409
CAS No.:72835-26-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

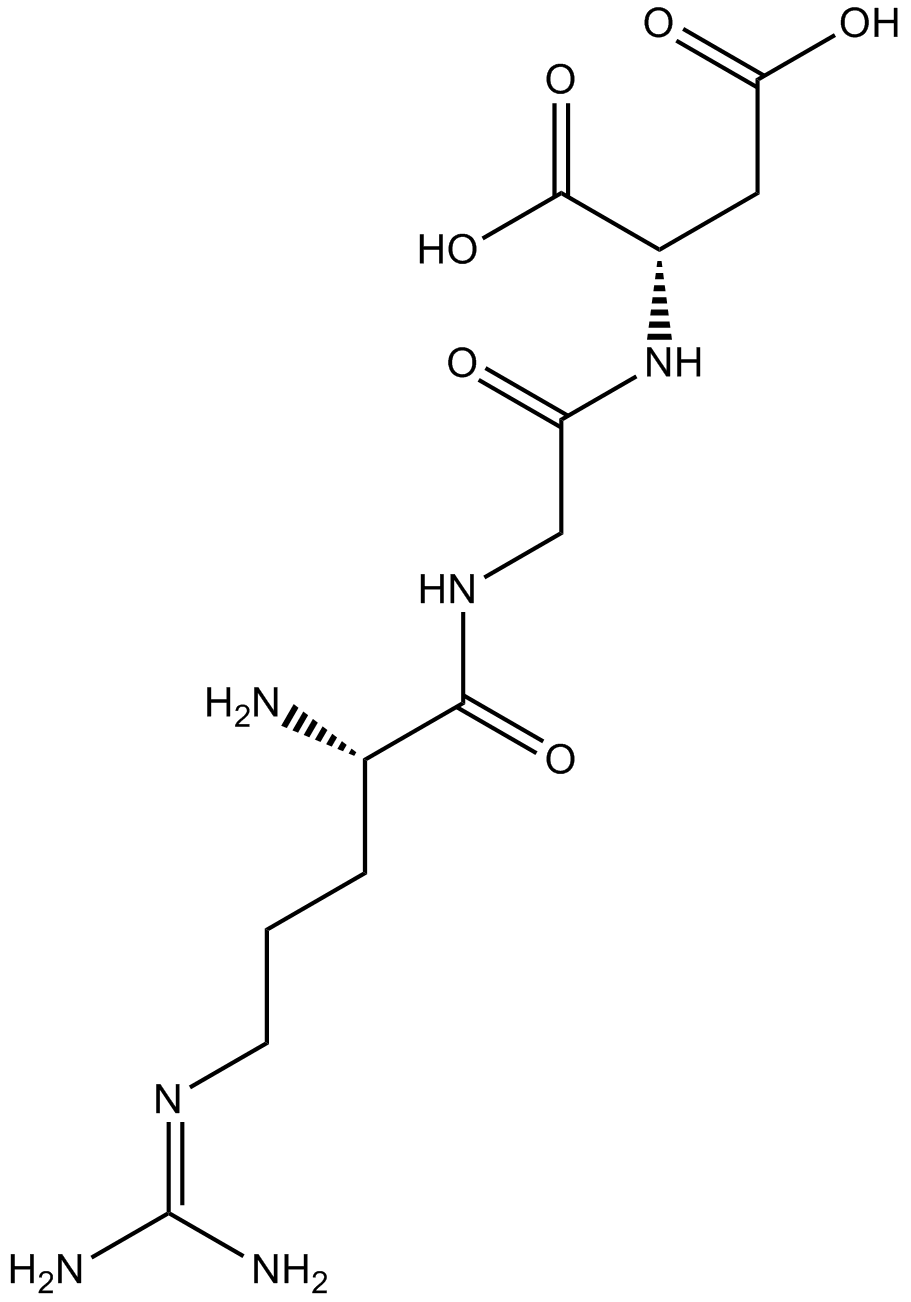
| Cas No. | 99896-85-2 | SDF | Download SDF |
| PubChem ID | 104802 | Appearance | Powder |
| Formula | C12H22N6O6 | M.Wt | 346.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 12 mg/mL (34.64 mM) in Water | ||
| Chemical Name | (2S)-2-[[2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]acetyl]amino]butanedioic acid | ||
| SMILES | C(CC(C(=O)NCC(=O)NC(CC(=O)O)C(=O)O)N)CN=C(N)N | ||
| Standard InChIKey | IYMAXBFPHPZYIK-BQBZGAKWSA-N | ||
| Standard InChI | InChI=1S/C12H22N6O6/c13-6(2-1-3-16-12(14)15)10(22)17-5-8(19)18-7(11(23)24)4-9(20)21/h6-7H,1-5,13H2,(H,17,22)(H,18,19)(H,20,21)(H,23,24)(H4,14,15,16)/t6-,7-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | RGD is an inhibitor of integrin-ligand interactions. | |||||
| Targets | integrin-ligand interaction | |||||

RGD (Arg-Gly-Asp) Peptides Dilution Calculator

RGD (Arg-Gly-Asp) Peptides Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Synthetic peptides containing the arginine-glycine-aspartate (RGD) were extensively used as inhibitors of integrin-ligand interactions in studies of cell adhesion, migration, growth and differentiation, since the RGD motif is an integrin-recognition motif found in many ligands.
In vitro: RGD peptide can induce apoptosis in the absence of signals and integrin-mediated cell clustering. Previous study demonstrates that RGD peptides promote apoptosis through activation of conformation changes enhancing pro-caspase-3 activation and autoprocessing [1].
In vivo: Anima study suggested that the RGD-4C-FITC-peptide bound to both endothelial and tumor cells in vivo and that peptide targeting should allow the delivery of therapeutic drugs to both endothelial and tumor cells [2].
Clinical trials: Currenlty no clinical data are available.
References:
[1] Nature. 1999 Feb 11;397(6719):534-9.
RGD peptides induce apoptosis by direct caspase-3 activation.
Buckley CD1, Pilling D, Henriquez NV, Parsonage G, Threlfall K, Scheel-Toellner D, Simmons DL, Akbar AN, Lord JM, Salmon M.
[2] Cancer Res. 2002 Sep 15;62(18):5139-43. Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Zitzmann S1, Ehemann V, Schwab M.
- Isoboonein acetate
Catalog No.:BCN4542
CAS No.:99891-77-7
- Kaempferol 3-O-arabinoside
Catalog No.:BCN4541
CAS No.:99882-10-7
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
- Rotigotine
Catalog No.:BCC1907
CAS No.:99755-59-6
- Scholaricine
Catalog No.:BCN4539
CAS No.:99694-90-3
- 14-Benzoylneoline
Catalog No.:BCN6493
CAS No.:99633-05-3
- Ro 19-4603
Catalog No.:BCC7228
CAS No.:99632-94-7
- Uncinatone
Catalog No.:BCN4547
CAS No.:99624-92-7
- ent-3beta,18-Dihydroxylabda-8(17),13E-dien-15-oic acid
Catalog No.:BCN7669
CAS No.:99624-39-2
- Kazinol A
Catalog No.:BCN3388
CAS No.:99624-28-9
- Kazinol B
Catalog No.:BCN4538
CAS No.:99624-27-8
- Isothymonin
Catalog No.:BCN3393
CAS No.:99615-01-7
- 3-(3,4-Dihydroxyphenyl)-1-n-propylpyrrolidine hydrobromide
Catalog No.:BCN8535
CAS No.:99933-30-9
- Bullatantriol
Catalog No.:BCN4543
CAS No.:99933-32-1
- Isoboonein
Catalog No.:BCN4545
CAS No.:99946-04-0
- Z-VDVAD-FMK
Catalog No.:BCC1138
CAS No.:N/A
- Acetyl Perisesaccharide C
Catalog No.:BCN8666
CAS No.:110764-09-5
- Dihydroartemisinic acid
Catalog No.:BCN8547
CAS No.:85031-59-0
- Rhein-8-glucoside
Catalog No.:BCN8548
CAS No.:34298-86-7
- Cistanoside F
Catalog No.:BCN8549
CAS No.:97411-47-7
- Vinaginsenoside R3
Catalog No.:BCN8550
CAS No.:156012-92-9
- 28-Demethyl-beta-amyrone
Catalog No.:BCN8551
CAS No.:73493-60-4
- Ilexsaponin B2
Catalog No.:BCN8552
CAS No.:108906-69-0
- Trans sodium crocetinate
Catalog No.:BCN8553
CAS No.:591230-99-8
Vasomotor effects of arg-gly-asp (RGD) peptides are limited and not related to endothelium-derived hyperpolarizing factor-mediated relaxation in rat mesenteric arteries.[Pubmed:11703386]
Clin Exp Pharmacol Physiol. 2001 Nov;28(11):873-6.
1. In the present study we tested the effect of arg-gly-asp (RGD) peptides on vasomotor responses in rat isolated mesenteric arteries. More specifically, the hypothesis was tested that RGD interaction with integrins mediates relaxation attributed to endothelium-derived hyperpolarizing factor (EDHF). 2. The presence of the beta3 integrin subunit was shown by western blot analysis. To study its functional role, arteries (355 +/- 11 microm; n = 50) were mounted in a wire myograph set-up to measure isometric force generation. After blockade of nitric oxide synthesis with N(G)-nitro-L-arginine (0.1 mmol/L) and prostaglandin synthesis with indomethacin (10 micromol/L), methacholine (10 micromol/L) induced a transient relaxation within 1 min of 72 +/- 4.0% (as percentage of precontraction with phenylephrine; n = 27). 3. These responses were inhibited by a 60 mmol/L potassium buffer (18 +/- 6.0%; n = 6) or endothelium denudation (12 +/- 3.2%; n = 7), consistent with EDHF. 4. A function-blocking monoclonal antibody against the integrin beta3 chain did not affect relaxation. 5. The RGD peptides gly-arg-gly-asp-thr-pro (GRGDTP), gly-arg-gly-asp-ser (GRGDS) and cyclic RGD, ligands for the RGD binding site of integrins, also did not affect relaxation induced by methacholine. 6. Cyclic RGD increased contraction from 91 +/- 3 to 98 +/- 3% (as percentage of 120 mmol/L potassium). 7. In conclusion, these data show that vasomotor responses related to integrins are small and not involved in hyperpolarization attributed to EDHF in rat mesenteric artery.
Age-related loss of rooting capability in Arabidopsis thaliana and its reversal by peptides containing the Arg-Gly-Asp (RGD) motif.[Pubmed:11975735]
Physiol Plant. 2002 Apr;114(4):601-607.
We describe here an experimental system to study the age-related decline of adventitious root formation in Arabidopsis thaliana L. (Heynh), ecotype Landsberg erecta (Ler). The system is based on the different rooting capacity of hypocotyls from de-rooted juvenile (12-day-old) and adult (26-day-old) plants. Hypocotyls from de-rooted juvenile plants rooted readily within a week of culture, and the rooting process was not dependent on exogenous auxin. In contrast, hypocotyls from de-rooted adult plants rooted poorly and only after longer periods of time. Exogenously applied auxin had no effect on rooting of hypocotyls from de-rooted adult plants. Rooting capacity, although correlated with the transition to flowering, did not depend on this transition. Root induction declined in a similar manner when the transition to flowering was delayed, either genetically with the fve mutant or physiologically with short days. The results showed that rooting of hypocotyls from de-rooted adult plants depended on the effect of peptides containing the RGD motif. Both the percentage of rooting and the number of roots were largely increased when the hypocotyls were treated transiently with the RGD peptide. The effect of the RGD peptide was a necessary, but not sufficient, condition for rooting of hypocotyls from de-rooted adult plants.
Arg-Gly-Asp (RGD)-containing peptides increase soluble guanylate cyclase in contractile cells.[Pubmed:16360131]
Cardiovasc Res. 2006 Feb 1;69(2):359-69.
OBJECTIVES: Alterations in NO/cGMP signaling have been associated with vascular dysfunction. Here, we tested whether peptides containing arginine-glycine-aspartic acid (RGD) motifs, commonly found on the binding sites of extracellular matrix to integrins, could increase the expression and function of soluble guanylate cyclase (sGC) in human mesangial cell (HMC), and human aortic smooth muscle (HASMC) cells. METHODS AND RESULTS: Arginine-glycine-aspartic acid-serine (RGDS) promoted an up-regulation in the sGC beta1 subunit steady-state level, both in HMC and HASMC, in a time- and dose-dependent manner. The cellular effects of RGDS-stimulation of sGC expression was an enhanced cellular response to sodium nitroprusside, resulting in elevated cGMP levels and vasodilator-stimulated phosphoprotein (VASP) phosphorylation in both kinds of cells, and an increased NO relaxing effect on cells precontracted with H(2)O(2) or Angiotensin II. Moreover, RGDS was able to restore the sGC levels that had been previously decreased by long term exposure to NO donors. RGDS effects on sGC regulation were due to the specific interaction with alpha(5)beta(1) integrin. To investigate the intracellular mechanisms activated after RGDS cell treatment, pharmacological kinase inhibitors were used. The effect of RGDS on sGC protein content was completely abolished by treating the cells with c-Jun N-terminal kinase (JNK) inhibitors. In addition, c-fos and c-jun were found in the cell nuclei after RGDS treatment, suggesting that the RGDS effect could be mediated by the AP-1 transcription factor. CONCLUSION: Results provide evidence of a mechanism able to increase the sGC protein content linked to increased activity in contractile cells, not only in basal conditions, but also after the down-regulation of the receptor by its own substrate. Elucidation of this novel mechanism provides a rationale for future pharmacotherapy in certain vascular diseases.
Supramolecular hydrogels formed by the conjugates of nucleobases, Arg-Gly-Asp (RGD) peptides, and glucosamine.[Pubmed:22844343]
Soft Matter. 2012 Jul 28;8(28):7402-7407.
Here we report the generation of a novel class of supramolecular hydrogelators based on the integration of nucleobase, Arg-Gly-Asp (RGD) peptides, and glucosamine in a single molecule. These novel small molecule hydrogelators self-assemble in water to form stable supramolecular nanofibers/hydrogels and exhibit useful biostability. This approach provides a new opportunity for systematic exploration of the self-assembly of small biomolecules by varying any individual segment to generate a large array of supramolecular hydrogels for biological functions and for biomedical applications.


