Anemarrhenasaponin ICAS# 163047-21-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 163047-21-0 | SDF | Download SDF |
| PubChem ID | 101672279 | Appearance | Powder |
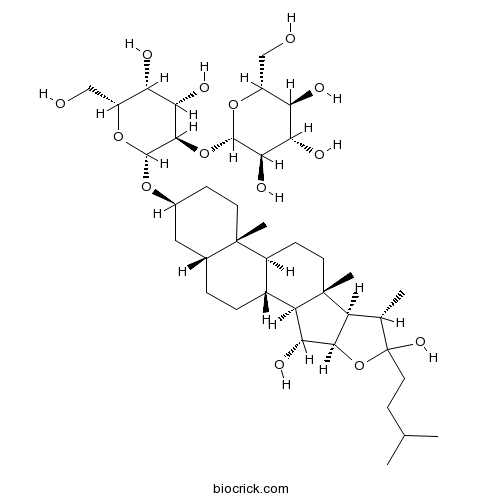
| Formula | C39H66O14 | M.Wt | 758.94 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (65.88 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5R,6R)-2-[[(1R,2S,3R,4R,7S,8R,9S,12S,13S,16S,18R)-3,6-dihydroxy-7,9,13-trimethyl-6-(3-methylbutyl)-5-oxapentacyclo[10.8.0.02,9.04,8.013,18]icosan-16-yl]oxy]-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC1C2C(C(C3C2(CCC4C3CCC5C4(CCC(C5)OC6C(C(C(C(O6)CO)O)O)OC7C(C(C(C(O7)CO)O)O)O)C)C)O)OC1(CCC(C)C)O | ||
| Standard InChIKey | ZNTKLBZCLHHWHW-WPUQNTDPSA-N | ||
| Standard InChI | InChI=1S/C39H66O14/c1-17(2)8-13-39(48)18(3)25-33(53-39)29(44)26-21-7-6-19-14-20(9-11-37(19,4)22(21)10-12-38(25,26)5)49-36-34(31(46)28(43)24(16-41)51-36)52-35-32(47)30(45)27(42)23(15-40)50-35/h17-36,40-48H,6-16H2,1-5H3/t18-,19+,20-,21+,22-,23+,24+,25-,26+,27+,28-,29+,30-,31-,32+,33+,34+,35-,36+,37-,38+,39?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Anemarrhenasaponin I shows remarkable inhibiting effect on platelet aggregation. |

Anemarrhenasaponin I Dilution Calculator

Anemarrhenasaponin I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3176 mL | 6.5881 mL | 13.1763 mL | 26.3525 mL | 32.9407 mL |
| 5 mM | 0.2635 mL | 1.3176 mL | 2.6353 mL | 5.2705 mL | 6.5881 mL |
| 10 mM | 0.1318 mL | 0.6588 mL | 1.3176 mL | 2.6353 mL | 3.2941 mL |
| 50 mM | 0.0264 mL | 0.1318 mL | 0.2635 mL | 0.5271 mL | 0.6588 mL |
| 100 mM | 0.0132 mL | 0.0659 mL | 0.1318 mL | 0.2635 mL | 0.3294 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydropalmatine
Catalog No.:BCN8558
CAS No.:26067-60-7
- Rupestonic acid
Catalog No.:BCN8557
CAS No.:115473-63-7
- Uvarigranol B
Catalog No.:BCN8556
CAS No.:164204-79-9
- Ginsenoside Ra2
Catalog No.:BCN8555
CAS No.:83459-42-1
- Panasenoside
Catalog No.:BCN8554
CAS No.:31512-06-8
- Trans sodium crocetinate
Catalog No.:BCN8553
CAS No.:591230-99-8
- Ilexsaponin B2
Catalog No.:BCN8552
CAS No.:108906-69-0
- 28-Demethyl-beta-amyrone
Catalog No.:BCN8551
CAS No.:73493-60-4
- Vinaginsenoside R3
Catalog No.:BCN8550
CAS No.:156012-92-9
- Cistanoside F
Catalog No.:BCN8549
CAS No.:97411-47-7
- Rhein-8-glucoside
Catalog No.:BCN8548
CAS No.:34298-86-7
- Dihydroartemisinic acid
Catalog No.:BCN8547
CAS No.:85031-59-0
- Glabrolide
Catalog No.:BCN8560
CAS No.:10401-33-9
- Acanthopanaxoside B
Catalog No.:BCN8561
CAS No.:915792-03-9
- Erigoster B
Catalog No.:BCN8562
CAS No.:849777-61-3
- 3'-Demethylnobiletin
Catalog No.:BCN8563
CAS No.:112448-39-2
- Lancifodilactone F
Catalog No.:BCN8564
CAS No.:850878-47-6
- Periplocoside N
Catalog No.:BCN8565
CAS No.:39946-41-3
- 3-Feruloyl-1-Sinapoyl sucrose
Catalog No.:BCN8566
CAS No.:98942-06-4
- Vinaginsenoside R8
Catalog No.:BCN8567
CAS No.:156042-22-7
- Saikogenin D
Catalog No.:BCN8568
CAS No.:5573-16-0
- Gancaonin N
Catalog No.:BCN8569
CAS No.:129145-52-4
- Graveobioside A
Catalog No.:BCN8570
CAS No.:506410-53-3
- 2-Amino-3-carboxy-1,4-naphthoquinone
Catalog No.:BCN8571
CAS No.:173043-38-4
Steroidal saponins from Anemarrhena asphodeloides and their effects on superoxide generation.[Pubmed:10617410]
Planta Med. 1999 Oct;65(7):661-3.
A new steroidal saponin, timosaponin F, along with six known compounds was isolated from the rhizomes of Anemarrhena asphodeloides Bge. On the basis of chemical and spectroscopic evidence, the structure of timosaponin F was elucidated as (5beta, 25 S):-spirostan-3beta,15alpha,23alpha-triol-3-O-beta- glucopyranosyl-(1--->2)-beta-galactopyranoside. The six known compounds were Anemarrhenasaponin I, Anemarrhenasaponin Ia, timosaponin BI, timosaponin BII, timosaponin B, timosaponin AIII; their effects on superoxide generation are also reported.
[Effects of different processing methods on five main chemical constituents of Anemarrhena asphodeloides Bge. studied by high performance liquid chromatography].[Pubmed:23593885]
Se Pu. 2012 Dec;30(12):1271-5.
A high performance liquid chromatographic (HPLC) method has been developed for the simultaneous determination of neomangiferin, mangiferin, timosaponin B III, Anemarrhenasaponin I and timosaponin A III in the products of Anemarrhena asphodeloides Bge. processed by different methods. By comparing and analyzing the variation of the contents of the five components, the effects of processing on chemical constituents of Anemarrhena asphodeloides Bge. were explored, which provided evidences for the relevance between processing and the property changes of Anemarrhena asphodeloides Bge. The chromatographic separation was performed on an Alltima C18 column (250 mm x 4.6 mm, 5 microm) with a gradient elution of acetonitrile (A) and 0. 1% formic acid (B) (0 - 10 min, 5% A - 20% A; 10 - 15 min, 20% A - 25% A; 15 -30 min, 25% A - 80% A; 30 -35 min, 80% A - 100% A) at a flow rate of 0.8 mL/min. Neomangiferin and mangiferin were detected by an ultraviolet detector at 265 nm and room temperature. Timosaponin B III, Anemarrhenasaponin I and timosaponin A III were detected by an evaporative light scattering detector with the drift temperature at 50 degrees C and gas pressure at 179.1 kPa (26 psi). To some extent, the contents of the major components varied in different processed products of Anemarrhena asphodeloides Bge. The results indicated that different processing methods caused significant differences in the contents of the major components of Anemarrhena asphodeloides Bge. It is of great use for further researching the relevance of the processing methods to pharmacodynamics of Anemarrhena asphodeloides Bge.
Effect of six steroidal saponins isolated from anemarrhenae rhizoma on platelet aggregation and hemolysis in human blood.[Pubmed:10556655]
Clin Chim Acta. 1999 Nov;289(1-2):79-88.
Six steroidal saponins were isolated from Anemarrhena asphodeloides Bunge (Liliaceae), a traditional chinese medicine, and named Anemarrhenasaponin I (An-I), Anemarrhenasaponin Ia (An-Ia), timosaponin B-I (TB-I), timosaponin B-II (TB-II), timosaponin B-III (TB-III), and timosaponin A-III (TA-III). The effects of these six compounds on platelet aggregation and hemolysis in human blood were studied. All these compounds provoked remarkable inhibiting effect on platelet aggregation, and activated partial thromboplastin times (APTT) are sensitive to the presence of these six compounds. Using an in vitro system, APTT was delayed with the increment of the concentrations of these six compounds. In these six compounds, only timosaponin A-III appeared a strong effect on hemolysis, and Anemarrhenasaponin Ia had a slight effect on hemolysis, other had no effect on hemolysis. These results suggested that these steroidal saponins isolated from Anemarrhena asphodeloides Bunge (Liliaceae) might be used as a novel antithrombotic therapeutic agents in post-myocardial infarction.
Steroidal glycosides from the rhizomes of Anemarrhena asphodeloides and their antiplatelet aggregation activity.[Pubmed:22307934]
Planta Med. 2012 Apr;78(6):611-6.
Five new steroidal glycosides, timosaponin J ( 1), timosaponin K ( 2), (25 S)-karatavioside C ( 5), timosaponin L ( 6), and (25 S)-officinalisnin-I ( 8), together with eight known steroidal saponins, timosaponin E (1) ( 3), purpureagitosid ( 4), timosaponin BII ( 7), timosaponin B III ( 9), Anemarrhenasaponin I ( 10), Anemarrhenasaponin III ( 11), anemarrhenasaponin A (2) ( 12), and timosaponin A III ( 13), were isolated from the rhizomes of Anemarrhena asphodeloides. Their structures were elucidated on the basis of spectroscopic and chemical evidence. The aglycones of compounds 1 and 2 are new aglycones. Compounds 1- 13 were evaluated for their platelet aggregation activities, and compound 13 exhibited the strongest inhibitory effect on adenosine diphosphate (ADP)-induced platelet aggregation.


