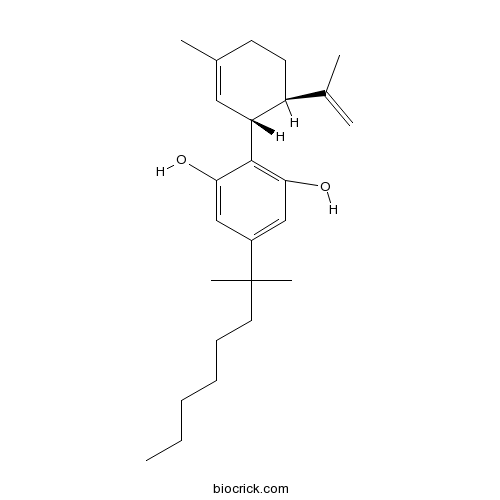
(-)-5'-DMH-CBDMetabolically stable anandamide transport inhibitor CAS# 97452-63-6 |
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Hydrochlorothiazide
Catalog No.:BCC4786
CAS No.:58-93-5
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
- TPT-260
Catalog No.:BCC5171
CAS No.:769856-81-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 97452-63-6 | SDF | Download SDF |
| PubChem ID | 126670 | Appearance | Powder |
| Formula | C25H38O2 | M.Wt | 370.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (-)-5'-Dimethylheptyl-cannabidiol | ||
| Solubility | Soluble in ethanol (supplied pre-dissolved in anhydrous ethanol, 5mg/ml) | ||
| Chemical Name | 5-(2-methyloctan-2-yl)-2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]benzene-1,3-diol | ||
| SMILES | CCCCCCC(C)(C)C1=CC(=C(C(=C1)O)C2C=C(CCC2C(=C)C)C)O | ||
| Standard InChIKey | MPJURNPNPDQYSY-LEWJYISDSA-N | ||
| Standard InChI | InChI=1S/C25H38O2/c1-7-8-9-10-13-25(5,6)19-15-22(26)24(23(27)16-19)21-14-18(4)11-12-20(21)17(2)3/h14-16,20-21,26-27H,2,7-13H2,1,3-6H3/t20-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Anandamide membrane transport inhibitor (IC50 = 14 μM) that is relatively metabolically stable. Displays some affinity for CB2 receptors but has only weak affinity for CB1 receptors and has no activity at VR1 receptors or FAAH. Anticonvulsive in vivo following systemic administration. |

(-)-5'-DMH-CBD Dilution Calculator

(-)-5'-DMH-CBD Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6985 mL | 13.4927 mL | 26.9855 mL | 53.9709 mL | 67.4636 mL |
| 5 mM | 0.5397 mL | 2.6985 mL | 5.3971 mL | 10.7942 mL | 13.4927 mL |
| 10 mM | 0.2699 mL | 1.3493 mL | 2.6985 mL | 5.3971 mL | 6.7464 mL |
| 50 mM | 0.054 mL | 0.2699 mL | 0.5397 mL | 1.0794 mL | 1.3493 mL |
| 100 mM | 0.027 mL | 0.1349 mL | 0.2699 mL | 0.5397 mL | 0.6746 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Mandelic acid benzyl ester
Catalog No.:BCC8374
CAS No.:97415-09-3
- 2',3'-Dehydrosalannol
Catalog No.:BCN4549
CAS No.:97411-50-2
- Tanshindiol A
Catalog No.:BCN3123
CAS No.:97411-46-6
- Cynatratoside A
Catalog No.:BCN7087
CAS No.:97399-96-7
- Paniculidine C
Catalog No.:BCN4524
CAS No.:97399-95-6
- Paniculidine B
Catalog No.:BCN4523
CAS No.:97399-94-5
- Paniculidine A
Catalog No.:BCN4522
CAS No.:97399-93-4
- Aristolactam AIIIa
Catalog No.:BCN4521
CAS No.:97399-91-2
- Aristolactam AIa
Catalog No.:BCN4854
CAS No.:97399-90-1
- 3alpha-Hydroxytanshinone IIA
Catalog No.:BCN2496
CAS No.:97399-71-8
- 8-Hydroxy-4-cadinen-3-one
Catalog No.:BCN4520
CAS No.:97372-53-7
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
- Cycloart-22-ene-3,25-diol
Catalog No.:BCN4525
CAS No.:97456-49-0
- Tanshindiol B
Catalog No.:BCN3124
CAS No.:97465-70-8
- Tanshindiol C
Catalog No.:BCN3125
CAS No.:97465-71-9
- α-Methyl-5-hydroxytryptamine maleate
Catalog No.:BCC6696
CAS No.:97469-12-0
- Pericyclivine
Catalog No.:BCN3974
CAS No.:975-77-9
- Ceftibuten
Catalog No.:BCC5216
CAS No.:97519-39-6
- 3-O-Caffeoyloleanolic acid
Catalog No.:BCN3959
CAS No.:97534-10-6
- Quinelorane hydrochloride
Catalog No.:BCC7100
CAS No.:97548-97-5
- 6-Methylgenistein
Catalog No.:BCN6852
CAS No.:97575-49-0
- Canrenone
Catalog No.:BCC7626
CAS No.:976-71-6
- Isoscoparin-2''-Beta-D-glucopyranoside
Catalog No.:BCN7807
CAS No.:97605-25-9
- Eticlopride hydrochloride
Catalog No.:BCC7193
CAS No.:97612-24-3
Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide.[Pubmed:11606325]
Br J Pharmacol. 2001 Oct;134(4):845-52.
1. (-)-Cannabidiol (CBD) is a non-psychotropic component of Cannabis with possible therapeutic use as an anti-inflammatory drug. Little is known on the possible molecular targets of this compound. We investigated whether CBD and some of its derivatives interact with vanilloid receptor type 1 (VR1), the receptor for capsaicin, or with proteins that inactivate the endogenous cannabinoid, anandamide (AEA). 2. CBD and its enantiomer, (+)-CBD, together with seven analogues, obtained by exchanging the C-7 methyl group of CBD with a hydroxy-methyl or a carboxyl function and/or the C-5' pentyl group with a di-methyl-heptyl (DMH) group, were tested on: (a) VR1-mediated increase in cytosolic Ca(2+) concentrations in cells over-expressing human VR1; (b) [(14)C]-AEA uptake by RBL-2H3 cells, which is facilitated by a selective membrane transporter; and (c) [(14)C]-AEA hydrolysis by rat brain membranes, which is catalysed by the fatty acid amide hydrolase. 3. Both CBD and (+)-CBD, but not the other analogues, stimulated VR1 with EC(50)=3.2 - 3.5 microM, and with a maximal effect similar in efficacy to that of capsaicin, i.e. 67 - 70% of the effect obtained with ionomycin (4 microM). CBD (10 microM) desensitized VR1 to the action of capsaicin. The effects of maximal doses of the two compounds were not additive. 4. (+)-5'-DMH-CBD and (+)-7-hydroxy-5'-DMH-CBD inhibited [(14)C]-AEA uptake (IC(50)=10.0 and 7.0 microM); the (-)-enantiomers were slightly less active (IC(50)=14.0 and 12.5 microM). 5. CBD and (+)-CBD were also active (IC(50)=22.0 and 17.0 microM). CBD (IC(50)=27.5 microM), (+)-CBD (IC(50)=63.5 microM) and (-)-7-hydroxy-CBD (IC(50)=34 microM), but not the other analogues (IC(50)>100 microM), weakly inhibited [(14)C]-AEA hydrolysis. 6. Only the (+)-isomers exhibited high affinity for CB(1) and/or CB(2) cannabinoid receptors. 7. These findings suggest that VR1 receptors, or increased levels of endogenous AEA, might mediate some of the pharmacological effects of CBD and its analogues. In view of the facile high yield synthesis, and the weak affinity for CB(1) and CB(2) receptors, (-)-5'-DMH-CBD represents a valuable candidate for further investigation as inhibitor of AEA uptake and a possible new therapeutic agent.
Anticonvulsant effects of the (-) and (+)isomers of cannabidiol and their dimethylheptyl homologs.[Pubmed:7071126]
Pharmacology. 1982;24(3):141-6.
The anticonvulsant actions of the (-) and (+)isomers of cannabidiol and dimethylheptyl-cannabidiol were studied with the maximal electroconvulsive shock model in mice. The ratio of the times for hindlimb extension and fore limb flexion was recorded as an anticonvulsant index. All the cannabinoids were anticonvulsant. They also potentiated pentobarbitone sleeping time. The (+)isomer was more active than the (-)isomer. Possible mechanisms of actions on receptors or membranes are discussed.


