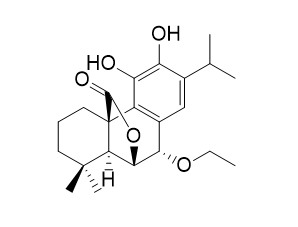
7-EthoxyrosmanolCAS# 111200-01-2 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 111200-01-2 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C22H30O5 | M.Wt | 374.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 7-Ethoxyrosmanol has cytotoxic and antimicrobial activities. |
| Targets | Antifection |
| In vitro | Cytotoxic and Antimicrobial Diterpenes Isolated from Hyptis Dilatata.[Reference: WebLink]Current Bioactive Compounds, 2015, 11(3):-.Diterpenes 7-Ethoxyrosmanol (1) and carnosol (2) were isolated and identified from the extracts of Hyptis dilatata collected in the Orinoco region, Colombia, used traditionally in this region for the treatment of wound infections of cattle. Their stereostructures were elucidated by ID and 2D NMR experiments including 1H, 13C, COSY, HSQC, HMBC and NOESY. Although compound 1 has been reported previously, its NMR assignments were revised, completed and corrected. These compounds showed a specific antimicrobial activity against Gram (+) strains. |
| Cell Research | Phenolic diterpenes derived from Hyptis incana induce apoptosis and G(2)/M arrest of neuroblastoma cells.[Pubmed: 23155243]Anticancer research, 2012, 32(11):4781-4789. Neuroblastoma is one of the most commonly encountered solid tumors in the pediatric age group, and the prognosis of patients with advanced neuroblastoma is very poor. In this study, the antitumor effects of five phenolic diterpenes derived from Hyptis incana (Lamiaceae), a Brazilian medicinal plant, were examined on neuroblastoma cells. |
| Structure Identification | Journal of Natural Products, 2002, 65(7):986-989.Semisynthesis of Rosmanol and Its Derivatives. Easy Access to AbietatrieneDiterpenes Isolated from the Genus Salvia with Biological Activities.[Pubmed: 12141857]
|

7-Ethoxyrosmanol Dilution Calculator

7-Ethoxyrosmanol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6702 mL | 13.3511 mL | 26.7023 mL | 53.4045 mL | 66.7557 mL |
| 5 mM | 0.534 mL | 2.6702 mL | 5.3405 mL | 10.6809 mL | 13.3511 mL |
| 10 mM | 0.267 mL | 1.3351 mL | 2.6702 mL | 5.3405 mL | 6.6756 mL |
| 50 mM | 0.0534 mL | 0.267 mL | 0.534 mL | 1.0681 mL | 1.3351 mL |
| 100 mM | 0.0267 mL | 0.1335 mL | 0.267 mL | 0.534 mL | 0.6676 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7,3',4',5'-Pentamethoxyflavone
Catalog No.:BCN8865
CAS No.:53350-26-8
- Isorhamnetin 7-O-glucoside
Catalog No.:BCN8864
CAS No.:6743-96-0
- 7-Methylcoumarin
Catalog No.:BCN8863
CAS No.:2445-83-2
- Isoarnebin I
Catalog No.:BCN8862
CAS No.:24502-79-2
- Pangelin
Catalog No.:BCN8861
CAS No.:33783-80-1
- 3beta-Methoxy-2,3-dihydrowithaferin A
Catalog No.:BCN8859
CAS No.:73365-94-3
- Hellebrigenin
Catalog No.:BCN8857
CAS No.:465-90-7
- Rhaponticin 6''-O-gallate
Catalog No.:BCN8856
CAS No.:94356-23-7
- Gardoside
Catalog No.:BCN8855
CAS No.:54835-76-6
- Rebaudioside E
Catalog No.:BCN8854
CAS No.:63279-14-1
- Neoastilbin
Catalog No.:BCN8853
CAS No.:54081-47-9
- 27-O-acetyl-withaferin A
Catalog No.:BCN8852
CAS No.:1214886-35-7
- Piperlonguminine
Catalog No.:BCN8867
CAS No.:5950-12-9
- Cassiaside B
Catalog No.:BCN8868
CAS No.:119170-51-3
- Emodin-1-O-beta-gentiobioside
Catalog No.:BCN8869
CAS No.:849789-95-3
- N-(p-Coumaroyl) serotonin
Catalog No.:BCN8870
CAS No.:68573-24-0
- Quercetin 3-O-sophoroside-7-O-rhamnoside
Catalog No.:BCN8871
CAS No.:64828-40-6
- Hydroxy-alpha-sanshool
Catalog No.:BCN8872
CAS No.:83883-10-7
- Aegeline
Catalog No.:BCN8873
CAS No.:456-12-2
- Caulophyllogenin
Catalog No.:BCN8874
CAS No.:52936-64-8
- N-trans-Sinapoyltyramine
Catalog No.:BCN8875
CAS No.:200125-11-7
- Sanguisorbigenin
Catalog No.:BCN8876
CAS No.:6812-98-2
- Benzoylgomisin P
Catalog No.:BCN8803
CAS No.:129445-43-8
- Silyamandin
Catalog No.:BCN8804
CAS No.:1009565-36-9
Investigation of In-Vitro Antioxidant and Electrochemical Activities of Isolated Compounds from Salvia chamelaeagnea P.J.Bergius Extract.[Pubmed:31013747]
Antioxidants (Basel). 2019 Apr 12;8(4). pii: antiox8040098.
We have investigated the in-vitro antioxidant activity and electrochemical redox properties of a number of natural compounds (carnosol, carnosic acid, 7-Ethoxyrosmanol, ursolic acid, rosmanol and ladanein) isolated from the methanolic extract of Salvia chamelaeagnea collected from the Cape floristic region, South Africa. The results from trolox equivalent antioxidant capacity (TEAC), ferric-ion reducing antioxidant parameter (FRAP) oxygen radical absorbance capacity (ORAC), as well as the inhibition of Fe(2+)-induced lipid peroxidation showed strong antioxidant capacities for carnosol and rosmanol. A structural analysis of the compounds suggests that multiple OH substitution, conjugation and lactone ring in carnosol and rosmanol are important determinants of the free radical scavenging activity and electrochemical behavior. Pharmacophore generated demonstrates H-donor/acceptor capabilities of the most active compounds. Rosmanol, when compared to other compounds, exhibits the lowest oxidation potential value with an anodic peak potential (Epa) value of 0.11 V, indicating that rosmanol has the highest antioxidant power, which is in good agreement with ORAC and lipid peroxidation experiments. The lipophilic nature of carnosol, carnosic acid and rosmanol enhanced their absorption and activity against oxidative stress related to the treatment of age-related diseases. These results confirm the first report on the in-vitro antioxidant and electrochemical activities of S. chamelaeagnea constituents and underline the medicinal uses of this plant as natural preservatives for skin ageing or in pharmaceutical applications.
Phenolic diterpenes derived from Hyptis incana induce apoptosis and G(2)/M arrest of neuroblastoma cells.[Pubmed:23155243]
Anticancer Res. 2012 Nov;32(11):4781-9.
BACKGROUND: Neuroblastoma is one of the most commonly encountered solid tumors in the pediatric age group, and the prognosis of patients with advanced neuroblastoma is very poor. In this study, the antitumor effects of five phenolic diterpenes derived from Hyptis incana (Lamiaceae), a Brazilian medicinal plant, were examined on neuroblastoma cells. MATERIALS AND METHODS: Cytotoxicity was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Apoptotic nuclear shrinkage was monitored by Hoechst 33342 staining. The cell-cycle status was evaluated by flow cytometry and protein alterations were monitored by western blotting. Differentiated cells were photographed and counted in a randomized fashion. RESULTS: All of the examined compounds exhibited significant cytotoxicity towards the neuroblastoma cells. In particular, 7-Ethoxyrosmanol had a high degree of efficacy. Nuclear condensation and degradation of procaspase-3 and -9 were observed after treatment of the cells with these compounds. Moreover, phenolic diterpenes induced cell-cycle arrest in the G(2)/M phase. Rosmanol and epirosmanol tended to induce differentiation. CONCLUSION: Phenolic diterpenes isolated from H. incana have multiple antitumor effects on neuroblastoma cells.
Semisynthesis of rosmanol and its derivatives. Easy access to abietatriene diterpenes isolated from the genus Salvia with biological activities.[Pubmed:12141857]
J Nat Prod. 2002 Jul;65(7):986-9.
The known diterpenes rosmanol (3), rosmaquinone (4), 7-methoxyrosmanol (5), 7-Ethoxyrosmanol (6), galdosol (7), and epirosmanol (8) have been obtained by partial synthesis from carnosol (2), an abundant natural product present in Salvia species. The physical and spectroscopic data of these semisynthetic diterpenes were identical to those of authentic natural samples and with data reported in the literature. These abietane diterpenes have very interesting biological activities and are present in the genus Salviain low quantities; thus, the semisynthetic approach described here represents an efficient alternative method to obtain these compounds. Additionally, the known diterpene 16-hydroxyrosmanol (10) and a new aromatic diterpene 11 were obtained from 16-hydroxycarnosol (9) by reaction with Ph3P/NBS in CH2Cl2. The structure of the new compound 11 was established from its spectroscopic data as 12,16-epoxycarnosol.


