N-trans-SinapoyltyramineCAS# 200125-11-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 200125-11-7 | SDF | Download SDF |
| PubChem ID | 25245053 | Appearance | Powder |
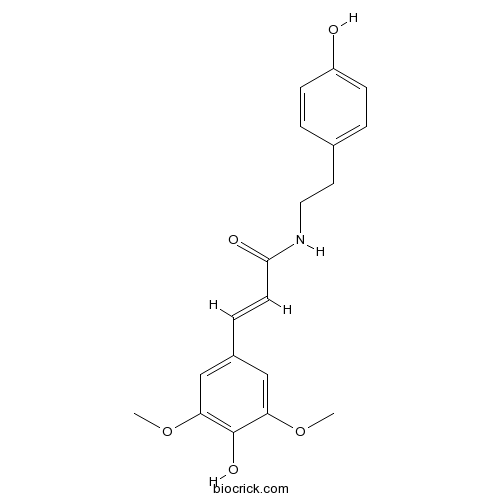
| Formula | C19H21NO5 | M.Wt | 343.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | trans-N-sinapoyltyramine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-(4-hydroxy-3,5-dimethoxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]prop-2-enamide | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C=CC(=O)NCCC2=CC=C(C=C2)O | ||
| Standard InChIKey | IEDBNTAKVGBZEP-VMPITWQZSA-N | ||
| Standard InChI | InChI=1S/C19H21NO5/c1-24-16-11-14(12-17(25-2)19(16)23)5-8-18(22)20-10-9-13-3-6-15(21)7-4-13/h3-8,11-12,21,23H,9-10H2,1-2H3,(H,20,22)/b8-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2018 Jan;43(1):109-113.Phenylpropanoid amides from whole plants of Corydalis edulis.[Pubmed: 29552819 ]
|

N-trans-Sinapoyltyramine Dilution Calculator

N-trans-Sinapoyltyramine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9121 mL | 14.5603 mL | 29.1206 mL | 58.2411 mL | 72.8014 mL |
| 5 mM | 0.5824 mL | 2.9121 mL | 5.8241 mL | 11.6482 mL | 14.5603 mL |
| 10 mM | 0.2912 mL | 1.456 mL | 2.9121 mL | 5.8241 mL | 7.2801 mL |
| 50 mM | 0.0582 mL | 0.2912 mL | 0.5824 mL | 1.1648 mL | 1.456 mL |
| 100 mM | 0.0291 mL | 0.1456 mL | 0.2912 mL | 0.5824 mL | 0.728 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Caulophyllogenin
Catalog No.:BCN8874
CAS No.:52936-64-8
- Aegeline
Catalog No.:BCN8873
CAS No.:456-12-2
- Hydroxy-alpha-sanshool
Catalog No.:BCN8872
CAS No.:83883-10-7
- Quercetin 3-O-sophoroside-7-O-rhamnoside
Catalog No.:BCN8871
CAS No.:64828-40-6
- N-(p-Coumaroyl) serotonin
Catalog No.:BCN8870
CAS No.:68573-24-0
- Emodin-1-O-beta-gentiobioside
Catalog No.:BCN8869
CAS No.:849789-95-3
- Cassiaside B
Catalog No.:BCN8868
CAS No.:119170-51-3
- Piperlonguminine
Catalog No.:BCN8867
CAS No.:5950-12-9
- 7-Ethoxyrosmanol
Catalog No.:BCN8866
CAS No.:111200-01-2
- 5,7,3',4',5'-Pentamethoxyflavone
Catalog No.:BCN8865
CAS No.:53350-26-8
- Isorhamnetin 7-O-glucoside
Catalog No.:BCN8864
CAS No.:6743-96-0
- 7-Methylcoumarin
Catalog No.:BCN8863
CAS No.:2445-83-2
- Sanguisorbigenin
Catalog No.:BCN8876
CAS No.:6812-98-2
- Benzoylgomisin P
Catalog No.:BCN8803
CAS No.:129445-43-8
- Silyamandin
Catalog No.:BCN8804
CAS No.:1009565-36-9
- (+)-δ-Tocopherol
Catalog No.:BCN8805
CAS No.:119-13-1
- 6-Hydroxykaempferol 3-beta-rutinoside
Catalog No.:BCN8807
CAS No.:205527-00-0
- Syringetin-3-O-rutinoside
Catalog No.:BCN8819
CAS No.:53430-50-5
- Quercetin 3-O-rutinoside-1-2-O-rhamnoside
Catalog No.:BCN8820
CAS No.:55696-57-6
- (3R)-5,7-Dihydroxy-6-methyl-3-(4'-hydroxybenzyl)chroman-4-one
Catalog No.:BCN8842
CAS No.:84638-48-2
- Loureiriol
Catalog No.:BCN8843
CAS No.:479195-44-3
- Hydroxy-γ-sanshool
Catalog No.:BCN8849
CAS No.:78886-66-5
- Astin A
Catalog No.:BCN8851
CAS No.:151201-75-1
- Astin B
Catalog No.:BCN8858
CAS No.:151201-76-2
Cytotoxic constituents from Isotrema tadungense.[Pubmed:32212861]
J Asian Nat Prod Res. 2020 Mar 26:1-7.
In our search for cytotoxic constituents from Vietnamese plants, the methanolic extract of Isotrema tadungense was found to exhibit significant cytotoxic effect. Subsequent phytochemical investigation of ethyl acetate fractions of this plant led to isolation of 11 compounds including one new arylbenzofuran rhamnoside namely aristolochiaside (1), two aristololactams (2 and 3), three lignanamides (4-6) and five phenolic amides (7-11). Their structures were elucidated by 1 D and 2 D NMR and HR-QTOF-MS experiments. Among the isolated compounds, aristolochiaside (1), aristolactam AIIIa (2) and N-trans-Sinapoyltyramine (10) exhibited strong and selective cytotoxicity on the HeLa human cancer cell line with IC50 values of 7.59 +/- 1.03, 8.51 +/- 1.73 and 9.77 +/- 1.25 muM, respectively.
[Phenylpropanoid amides from whole plants of Corydalis edulis].[Pubmed:29552819]
Zhongguo Zhong Yao Za Zhi. 2018 Jan;43(1):109-113.
Ten phenylpropanoid amides were isolated from the whole plants of Corydalis edulis Maxim. by various of column chromatographies including silica gel, Sephadex LH-20, and ODS. Their structures were identified on the basis of physicochemical properties, MS, NMR, and IR spectroscopic data. These compounds were identified as N-trans-sinapoyl-3-methoxytyramine-4'-O-beta-glucoside(1), N-trans-sinapoyl-3-methoxytyramine(2), N-trans-Sinapoyltyramine(3), N-trans-p-coumaroyltyramine(4), N-trans-sinapoyl-7-hydroxytyramine(5), N-cis-feruloyltyramine(6), N-cis-p-coumaroyltyramine(7), N-trans-feruloyltyramine(8), N-trans-feruloyl-3-methoxytyramine(9), and N-trans-feruloyl-7-hydroxytyramine(10). Compound 1 is a new compound. Compounds 2-7 are obtained from the plants of Papaveraceae for the first time, while compounds 8-10 are firstly isolated from C. edulis.
Phenolics, acyl galactopyranosyl glycerol, and lignan amides from Tetragonia tetragonioides (Pall.) Kuntze.[Pubmed:30263405]
Food Sci Biotechnol. 2016 Oct 31;25(5):1275-1281.
Eleven antioxidative compounds, including five lignin amides, were isolated from the aerial part of Tetragonia tetragonioides (New Zealand spinach) using 1,1-diphenyl-2-picrylhydrazyl radicalscavenging assay-guided purification. The structures were determined by nuclear magnetic resonance and electrospray ionization-mass spectroscopy. These compounds were identified as methyl linoleate (1), methyl coumarate (2), methyl ferulate (3), 1-O-stearoyl-3-O-beta-D-galactopyranosyl-sn-glycerol (4), 1-O-caffeoyl-beta-D-glucopyranoside (5), N-trans-caffeoyltyramine (6), cannabisin B (7), cannabisin A (8), Ntrans-feruloyltyramine (9), N-cis-feruloyltyramine (10), and N-trans-Sinapoyltyramine (11). Compounds 1, 2, 4, 5, and 8-11 were isolated for the first time from this plant.
[Amides from Peperomia tetraphylla].[Pubmed:20450045]
Zhongguo Zhong Yao Za Zhi. 2010 Feb;35(4):468-9.
OBJECTIVE: To investigate the chemical constituents of Peperomia tetraphylla. METHOD: The constituents of EtOAc-soluble portion were isolated and purified by chromatography. Their structures were identified by spectral features. RESULT: Six amides were isolated and identified as, aristololactam All (1), aristololactam B II (2), N-trans-feruloyltyramine (3), N-trans-Sinapoyltyramine (4), N-trans-feruloylmethoxytyramine (5), N-p-coumaroyltyramine (6). CONCLUSION: All compounds were isolated from this plant for the first time.


